Method for preparing 2-amino-3-methyl nitrobenzoate
A technology of methyl nitrobenzoate and methyl anthranilate, which is applied in the field of organic chemical synthesis, can solve the problems of long reaction time, cumbersome operation, and large potential safety hazard, achieve high value, improve production efficiency, and increase yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
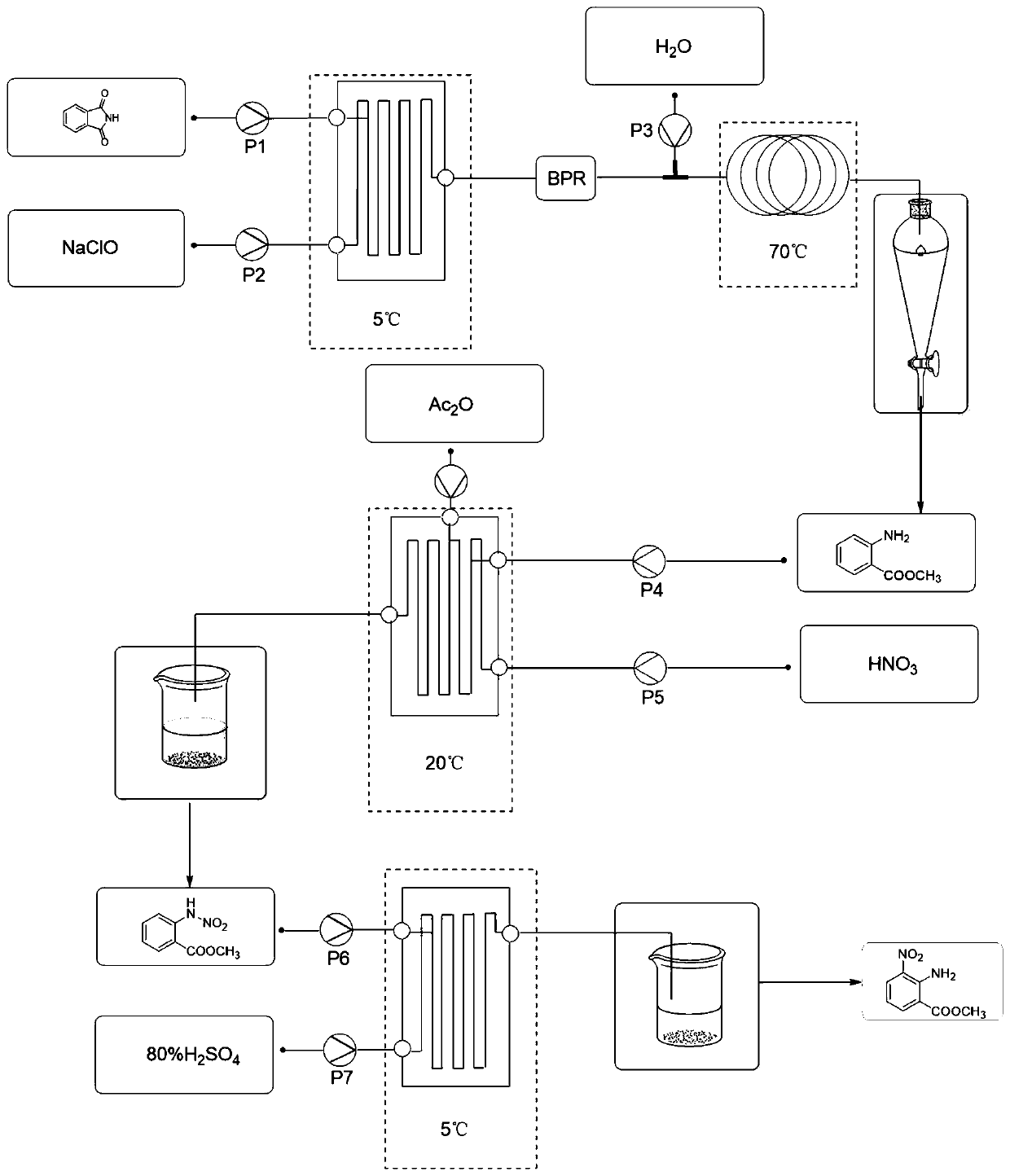
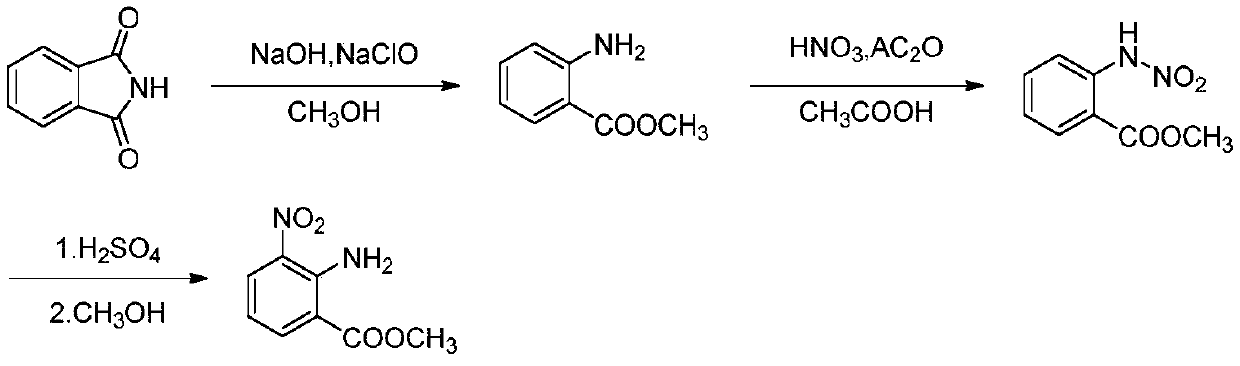
[0055] (1) Phthalimide, water, sodium hydroxide and methanol are prepared in a mass ratio of 1:1.05:0.15:0.9 to prepare a phthalimide solution. The phthalimide solution and the sodium hypochlorite solution are pumped into the microchannel reactor through the metering pump at flow rates of 26.84ml / min and 32.81ml / min respectively, the reaction temperature is -5°C, and the retention time of the reaction solution is 20s. The microreactor is connected in series with the pipeline reaction; at the same time, the water is preheated, the water pump is turned on, and the flow rate is 32.81ml / min. The retention time is 300s, and the reaction solution flows out, and the methyl anthranilate solution is obtained by continuous centrifugation in the ring gap type for future use. The purity determined by liquid chromatography is greater than 97.8%, and the yield is not less than 95.0%.
[0056] (2) The methyl anthranilate solution obtained in step 1 was diluted with glacial acetic acid to ob...
Embodiment 2
[0060] (1) Phthalimide, water, sodium hydroxide and methanol are formulated into a phthalimide solution in a mass ratio of 1:1.55:0.25:1.125. The phthalimide solution and the sodium hypochlorite solution are pumped into the microchannel reactor through the metering pump at flow rates of 15.50ml / min and 24.03ml / min respectively, the reaction temperature is 5°C, and the retention time of the reaction solution is 30s. The reactor is connected in series with the pipeline reaction; at the same time, the water is preheated, the water pump is turned on, and the flow rate is 24.03ml / min. After 180 s, the reaction liquid flows out, and the methyl anthranilate solution is obtained by continuous centrifugation in annulus for future use. Different batches of effluents were sampled, and the purity determined by liquid chromatography was greater than 98.2%, and the yield was not less than 94.6%.
[0061] (2) The methyl anthranilate solution obtained in step 1 was diluted with glacial aceti...
Embodiment 3
[0065] (1) Phthalimide, water, sodium hydroxide and methanol are prepared in a mass ratio of 1:2.05:0.35:1.35 to prepare a phthalimide solution. The phthalimide solution and the sodium hypochlorite solution are pumped into the microchannel reactor through the metering pump at the flow rate of 10.50ml / min and 18.90ml / min respectively, the reaction temperature is 15°C, and the retention time of the reaction solution is 40s. The reactor is connected in series with the pipeline reaction; at the same time, the water is preheated, the water pump is turned on, and the flow rate is 18.90ml / min. After 200 s, the reaction solution flows out, and the methyl anthranilate solution is obtained by continuous centrifugation in the ring gap type for subsequent use. Different batches of effluents were sampled, and the purity determined by liquid chromatography was greater than 99.4%, and the yield was not less than 95.6%.
[0066] (2) The methyl anthranilate solution obtained in step 1 was dil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com