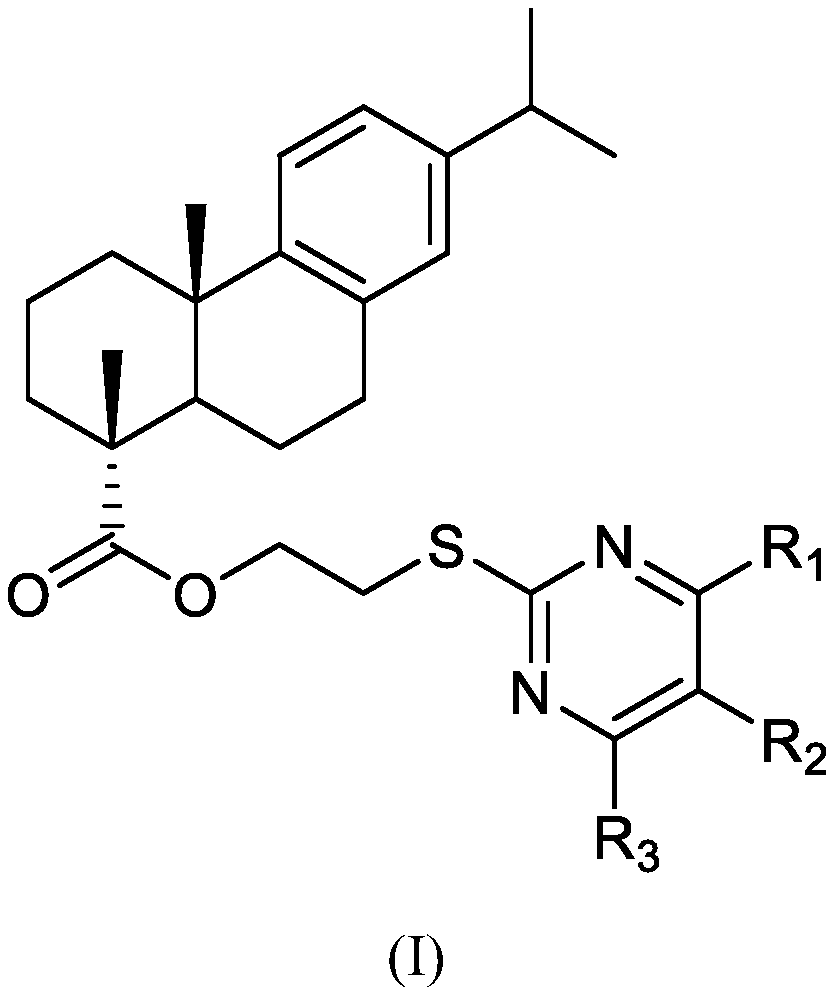
Pyrimidine dehydroabietate derivatives and preparation method and application thereof
A technology of hydroabietic acid pyrimidine and dehydroabietic acid, which is applied in the field of medicine, can solve the problems that there are no public reports of dehydroabietic acid pyrimidine derivatives, and achieve the effects of improving antitumor activity, stable quality, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
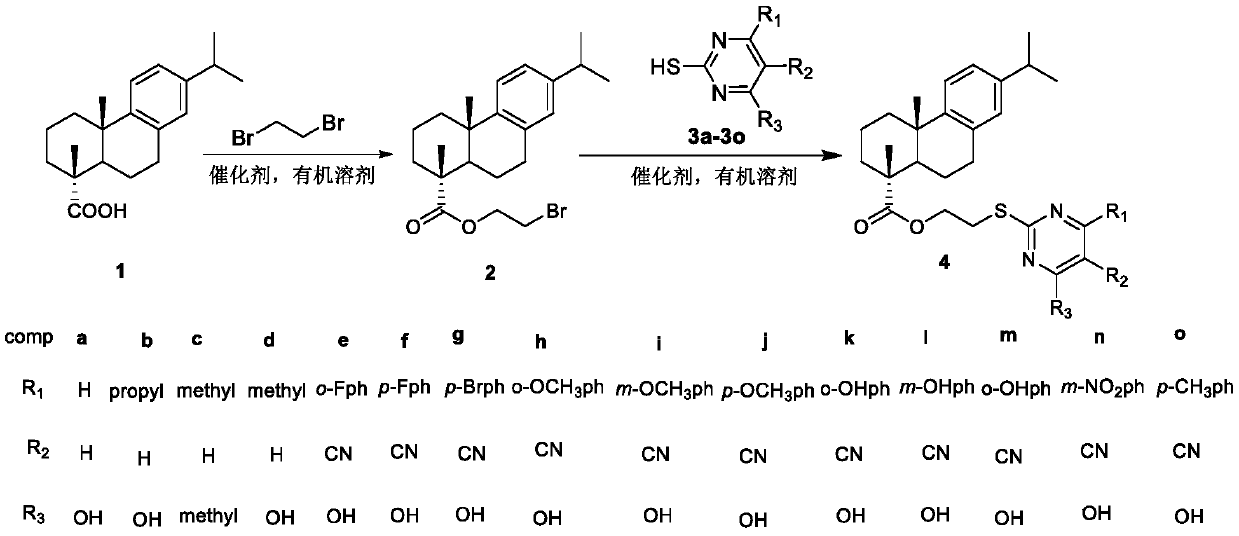
[0039] Preparation of bromoethyl dehydroabietate 2:
[0040] Dissolve 6.00g (20mmol) of dehydroabietic acid in 100ml of acetone, heat in a water bath, then add 5.52g (40mmol) of anhydrous potassium carbonate, and slowly add 5.2ml (60mmol) of dibromoethane dropwise, and react at a constant temperature of 60°C. TLC followed the progress of the reaction. After the reaction is complete, filter, distill and concentrate at 50°C to obtain a yellow oily liquid, purify by column chromatography, distill and concentrate, and vacuum dry to obtain a transparent oily liquid, which is cooled and condensed into 6.2 g of a white solid, with a yield of 78.6%; m.p.46.2 -49.4°C;
[0041] Therefore, above-mentioned compound 2 is bromoethyl dehydroabietate, and its structural formula is as shown in the following formula:
[0042]
Embodiment 2
[0044] Preparation of 2'-(4-hydroxypyrimidin-2-yl)mercapto dehydroabietic acid ethyl ester 4a:
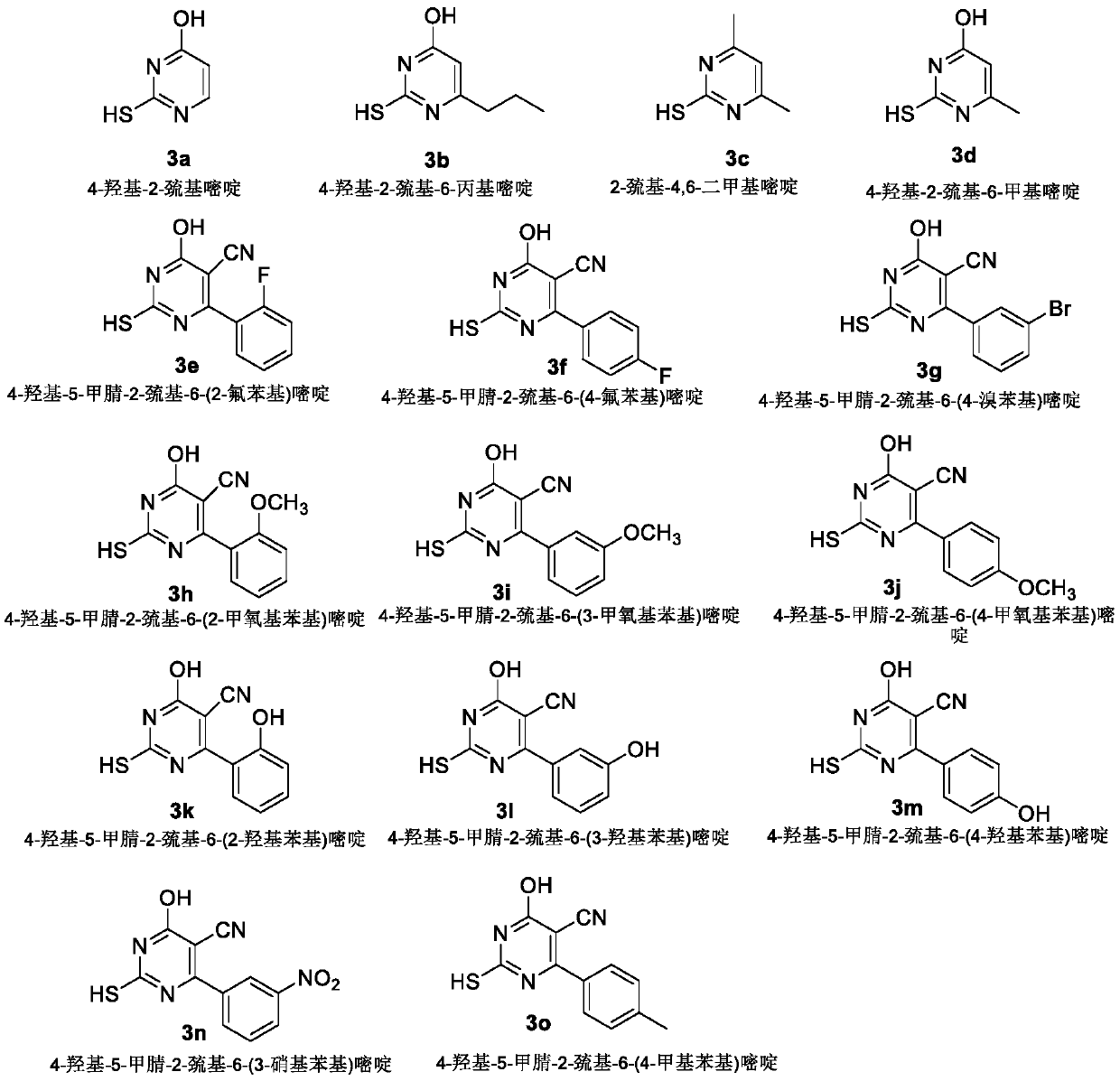
[0045] Dissolve 3mmol of pyrimidine compound 3a (4-hydroxy-2-mercaptopyrimidine), 3mmol (1.22g) of bromoethyl dehydroabietate in 20ml of DMF, then add 3mmol of anhydrous potassium carbonate, and react for 8-10 hours under stirring at room temperature , the progress of the reaction was monitored by TLC. After the reaction is complete, the reactant is poured into 100ml of ice water, and acidified with glacial acetic acid until no precipitation occurs; the precipitate is filtered, washed with water, dried and purified to obtain a light yellow solid with a yield of 56.1%, m.p.82.2~84.7°C ;
[0046] 1 H NMR (600MHz, CDCl 3 )δ7.81(d, J=6.6Hz, 1H), 7.28(s, 1H), 7.18(d, J=8.2Hz, 1H, H-11), 7.02(d, J=8.1Hz, 1H, H -12),6.90(s,1H,H-14),6.21(d,J=6.5Hz,1H),4.34(dd,J=31.8,8.7Hz,2H),3.45(d,J=15.9Hz, 2H),2.90(s,1H),2.84(s,1H),2.31(d,J=11.4Hz,1H),2.24(d,J=12.5Hz,1H),2.06(s,1H),1.83( d,J=5.4Hz...
Embodiment 3
[0050] Preparation of 2'-(4-hydroxyl-6-propylpyrimidin-2-yl)mercapto dehydroabietic acid ethyl ester (compound 4b):
[0051] Dissolve 3mmol of pyrimidine compound 3b (4-hydroxy-2-mercapto-6-propylpyrimidine) and 3mmol of bromoethyl dehydroabietate in 20ml of DMF, then add 3mmol of anhydrous potassium carbonate, and react for 8-10 hours under stirring at room temperature , the progress of the reaction was monitored by TLC. After the reaction is complete, the reactant is poured into 100ml of ice water, acidified with glacial acetic acid until no precipitation occurs; the precipitate is filtered, washed with water, dried and purified to obtain a white solid with a yield of 70.5%, m.p.69.8-71.3°C;
[0052] 1 H NMR (600MHz, CDCl 3 )δ7.18(d, J=8.2Hz, 1H, H-11), 7.02(d, J=8.0Hz, 1H, H-12), 6.89(s, 1H, H-14), 4.35(s, 1H),4.29(s,1H),3.39(s,1H),2.89(s,1H),2.83(s,1H),2.45(s,1H),2.44(s,1H),2.43(s,1H ),2.31(d,J=12.0Hz,1H),2.26(d,J=12.4Hz,1H),1.78(s,1H),1.77(s,1H),1.68(s,1H),1.67(s ,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com