A kind of non-combustible gel polymer electrolyte and its preparation method and application
A gel polymer and electrolyte technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve the problems of lithium battery combustion and explosion, achieve good compatibility, simple preparation process, and easy to achieve effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
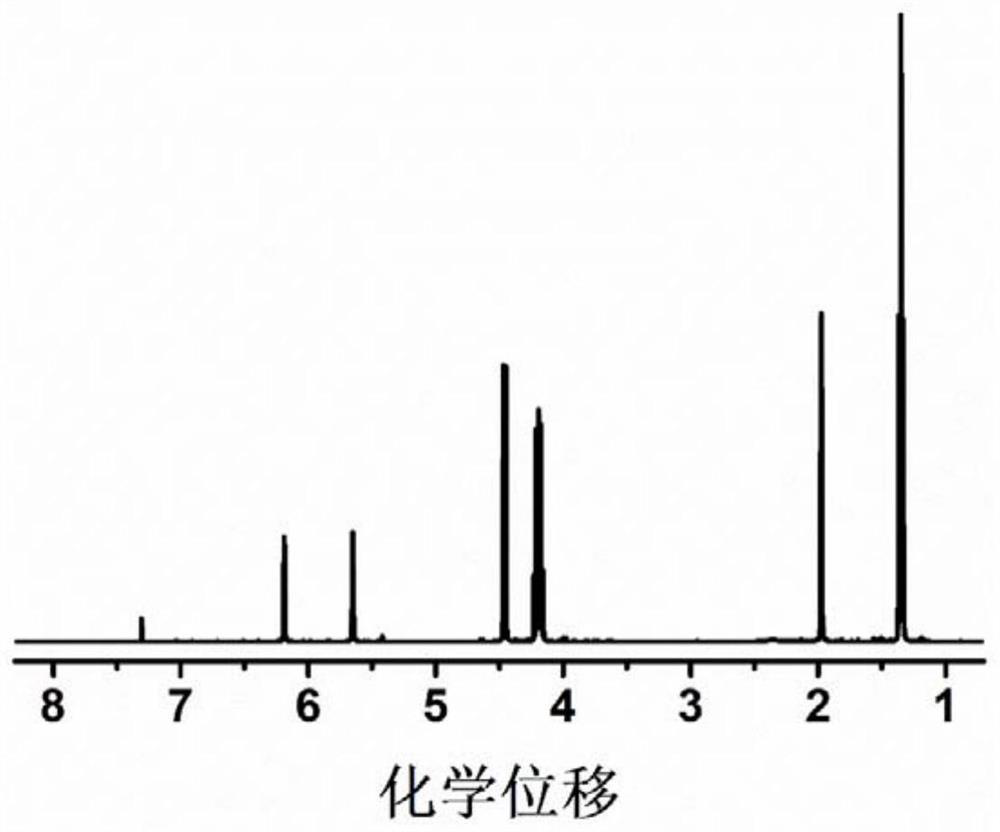
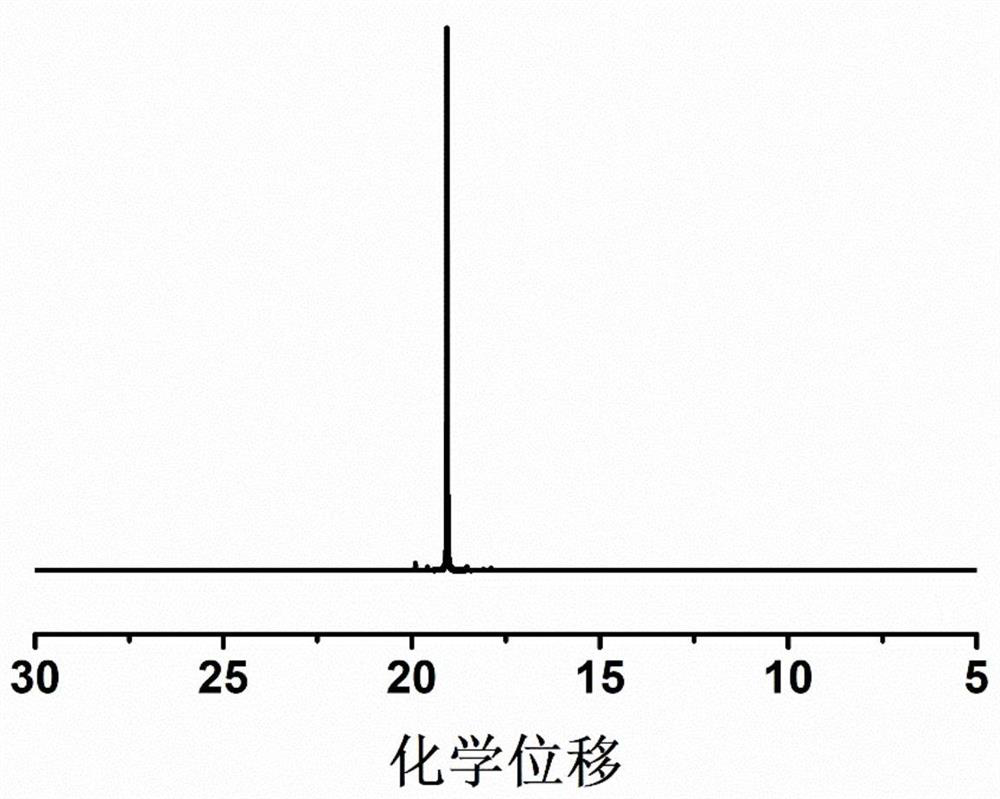
[0054] 4g of diethyl vinylphosphonate, 2g of acrylonitrile and 0.76g of LiPF 6 Dissolve in 5.234g EC and DMC (volume ratio 1:1), add 0.006g AIBN, stir until a homogeneous solution is formed, place it at 50°C for in-situ polymerization, and obtain a gel polymer electrolyte after 24 hours.
[0055] The prepared gel polymer is non-combustible in the ignition test, and the ion conductivity at room temperature is 2.5×10 -4 S cm -1 , the lithium ion migration number is 0.66, and the electrochemical window is 5.0V.
Embodiment 2
[0057] 4g of diethyl vinylphosphonate, 2g of acrylonitrile and 0.76g of LiPF 6 Dissolve in 5.234g EC and DMC (volume ratio 1:1), add 0.006g AIBN, stir until a homogeneous solution is formed, place it at 80°C for in-situ polymerization, and obtain a gel polymer electrolyte after 3 hours.
[0058] The prepared gel polymer is non-combustible in the ignition test, and the ion conductivity at room temperature is 2.8×10 -4 S cm -1 , the lithium ion migration number is 0.68, and the electrochemical window is 5.0V.
Embodiment 3
[0060] 4 g of diethyl vinylphosphonate, 2 g of methyl methacrylate and 0.76 g of LiPF 6 Dissolve in 5.234g EC and DMC (volume ratio 1:1), add 0.006g AIBN, stir until a homogeneous solution is formed, place it at 60°C for in-situ polymerization, and obtain a gel polymer electrolyte after 12 hours.
[0061] The prepared gel polymer is non-combustible in the ignition test, and the ion conductivity at room temperature is 1.9×10 -4 S cm -1, the lithium ion migration number is 0.38, and the electrochemical window is 4.2V.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com