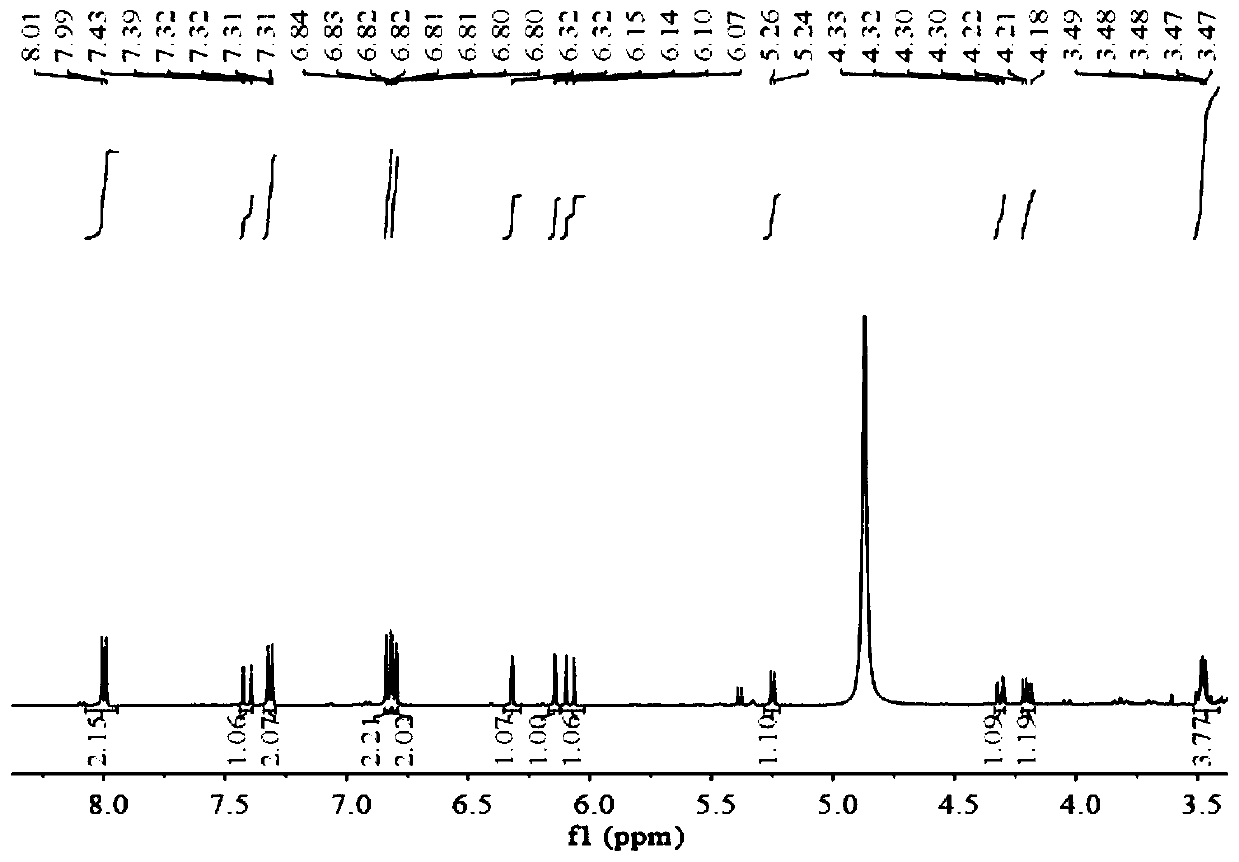
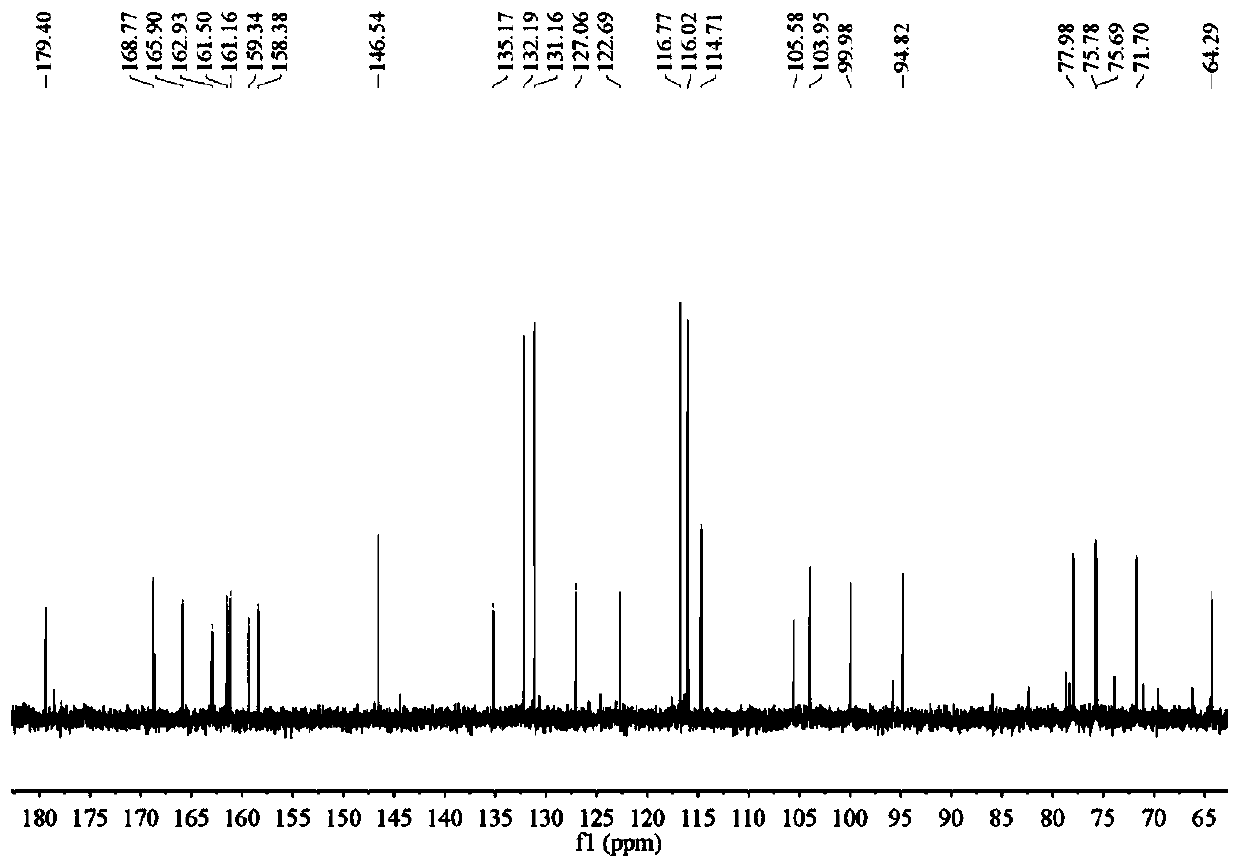
Application of tiliroside in preparation of anti-influenza drug
An anti-influenza drug, the technology of silver satin, applied in the field of chemical biology, can solve the problem that neuraminidase activity has not been reported, and achieve the effect of reducing replication and inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Determination of Inhibitory Activity of Linlin on Neuraminidase of Clostridium perfringens in Vitro
[0036] (1) Solution preparation
[0037] NA solution: An aliquot of NA freeze-dried powder is stored at -20°C. When in use, take a tube and dissolve it in MES buffer with a concentration of 0.021U / mL. Each dissolved NA is used on the same day.
[0038] Sample to be tested: Dissolve the sample in DMSO.
[0039] MES buffer: containing 32.5mmol / L MES, 4mmol / L CaCl 2 , adjust the pH to 6.5.
[0040] MUNANA: Use MES buffer to dissolve into 0.1mmol / L MUNANA solution.
[0041] (2) Measurement of NA activity using the fluorescent substrate MUNANA
[0042] The NA activity assay was carried out in a 384-well plate, and the sample addition method is shown in Table 2. The specific operation method is: the 384-well plate contains 25 μL diluted active NA solution and 25 μL mixed solution of fluorescent substrate and sample, and the concentration of fluorescent substrate in the mix...
Embodiment 2
[0054] In vitro Inhibitory Activity of Silver Lindenin on Neuraminidase of Oseltamivir-resistant / Sensitive Influenza Viruses
[0055] (1) Source of virus supernatant: A / Puerto Rico / 8 / 34(H1N1)(PR8) and its H274Y mutant strain, from the State Key Laboratory of Respiratory Diseases, Guangzhou Medical University.
[0056] The purified virus was lysed with 1% Triton in PBS buffer to obtain a NAase solution, and the virus protein concentration used was 1 mg / mL.
[0057] (2) Solution preparation
[0058] NA solution: According to the results of the preliminary experiment, select the appropriate concentration of the virus supernatant.
[0059] Sample to be tested: Dissolve the sample in phosphate Tween buffered saline (PBST) containing 0.2% tween20.
[0060] MES buffer: containing 33mmol / L MES, 4mmol / L CaCl 2 , adjust the pH to 3.5.
[0061] MUNANA: Use MES buffer to dissolve into 0.2mmol / L MUNANA solution.
[0062] (3) Measurement of NA activity using the fluorescent substrate M...
Embodiment 3
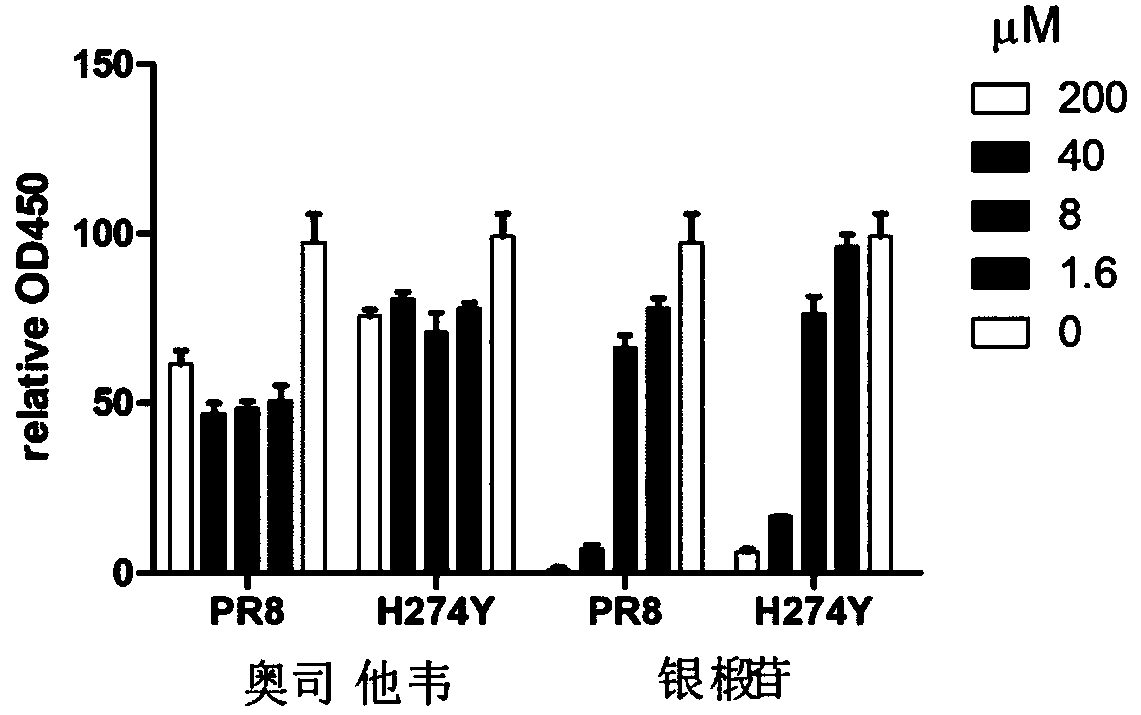
[0069] Inhibitory effect of silver lintin on the replication of oseltamivir-sensitive and drug-resistant influenza virus strains
[0070] MDCK cells were grown on a 96-well plate and infected with PR8 virus (oseltamivir-sensitive strain or resistant recombinant virus with H274Y mutation) at a multiplicity of infection (MOI) of 0.01 for 2 h (DMEM containing 0.05% bovine serum white protein and 1 μg / mL TPCK), and then the medium containing the virus was removed (wherein the medium refers to the DMEM medium containing 0.05% bovine serum albumin and 1 μg / mL TPCK), and then the medium containing 0, 1.6, 8 , 40, and 200 μM doses of satinin solution in the drug medium were added to the 96-well plate, and after continuing to culture for 24 hours, the virus-infected cells were fixed with 4% paraformaldehyde in PBS solution. Expression of viral proteins in cells was detected by enzyme-linked immunosorbent assay (ELISA) of anti-PR8 mouse serum. Inhibitory activity is shown as the relati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com