Tissue sealing agent powder and preparation process thereof, and tissue sealing agent
A sealing agent and tissue technology, applied in the field of biodegradable polymer materials, can solve the problems of inconsistent operation of the push rod, easy formation of thrombus, and blockage of the front end of the drug delivery device, so as to improve the instantaneous liquid suction ability, avoid related complications, The effect of preventing excessive formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
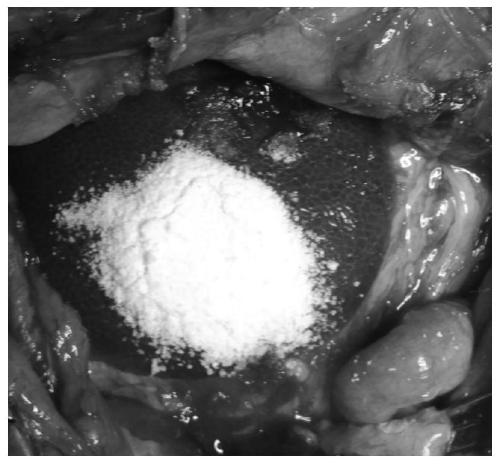
[0045] The physical melting method fully mixes PEG-SS (4, 10000Da) and 4 times the quality of PEG-SS super absorbent carboxymethyl starch sodium powder, and through granulation and other processes to obtain tissue sealant powder or hemostatic material, which is applied to animals with severe For bleeding wounds, press for 25s, solidify excess powder with physiological diluent, or rinse with a large amount of physiological diluent to stop bleeding. Physiological diluent is phosphate buffer (pH=7.4);
[0046] The preparation method of phosphate buffer solution with pH=7.4: Weigh 4.005g of sodium chloride, 0.1g of potassium chloride, 1.7907g of disodium hydrogen phosphate dodecahydrate, and 0.135g of potassium dihydrogen phosphate, and place them in a 500mL volumetric flask in turn , and then dissolved to obtain a PBS buffer solution with pH=7.4;
[0047] Tissue sealant powder (powder A + powder B): physiological diluent = 1:3.
Embodiment 2
[0049] The physical melting method fully mixes PEG-SC (2,5000Da) and 4 times the quality of PEG-SC super absorbent carboxymethyl starch sodium powder, and obtains tissue sealant powder through granulation and other processes, which is applied to severe bleeding wounds of animals. Press for 30 seconds to stop bleeding.
Embodiment 3
[0051] PEG-SPA (4, 10000Da) and 6 times the quality of PEG-SPA high water carboxymethyl starch sodium powder are fully mixed by physical melting method, and through granulation and other processes to obtain tissue sealant powder or hemostatic material, which is applied to animals with severe For bleeding wounds, press for 25s, solidify excess powder with physiological diluent, or rinse with a large amount of physiological diluent to stop bleeding. Physiological diluent is phosphate buffer (pH=7.4); tissue sealant powder (powder A+powder B): physiological diluent=1:3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com