Lithium metal negative electrode, preparation thereof and application of lithium metal negative electrode
A lithium metal negative electrode, lithium metal technology, applied in the direction of negative electrode, battery electrode, lithium battery, etc., can solve the problems of reducing lithium metal battery capacity, low ionic conductivity at room temperature, battery short circuit, etc., and achieve good electrochemical reaction activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
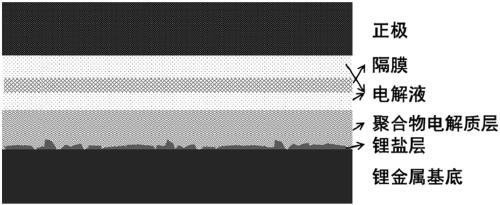
[0037] Lithium discs with a diameter of 16mm (with a surface area of 2cm 2 ) was uniformly covered with 0.6 g of lithium bisfluorosulfonate imide, and then 0.2 g of DOL was added dropwise to form a polymer electrolyte layer with a thickness of 20 μm. The thickness of the solid lithium salt layer is 10 μm.
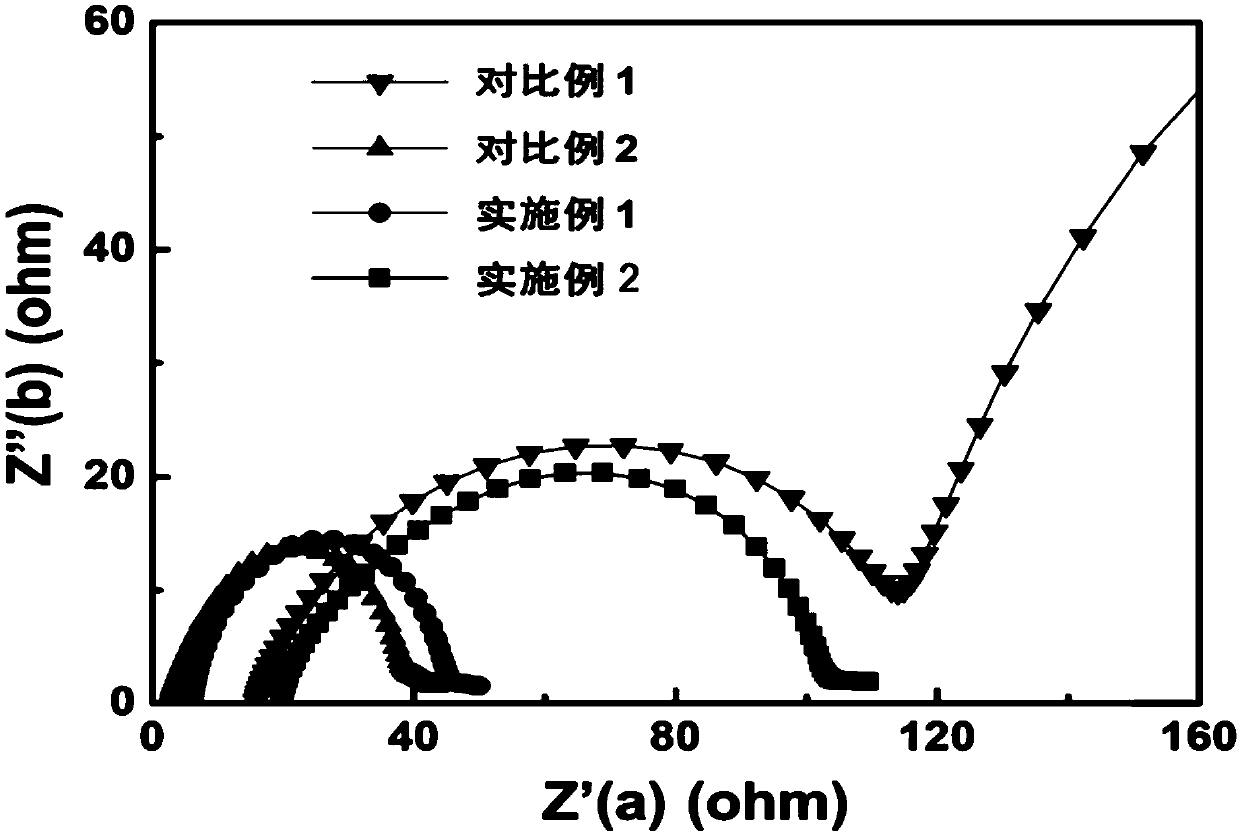
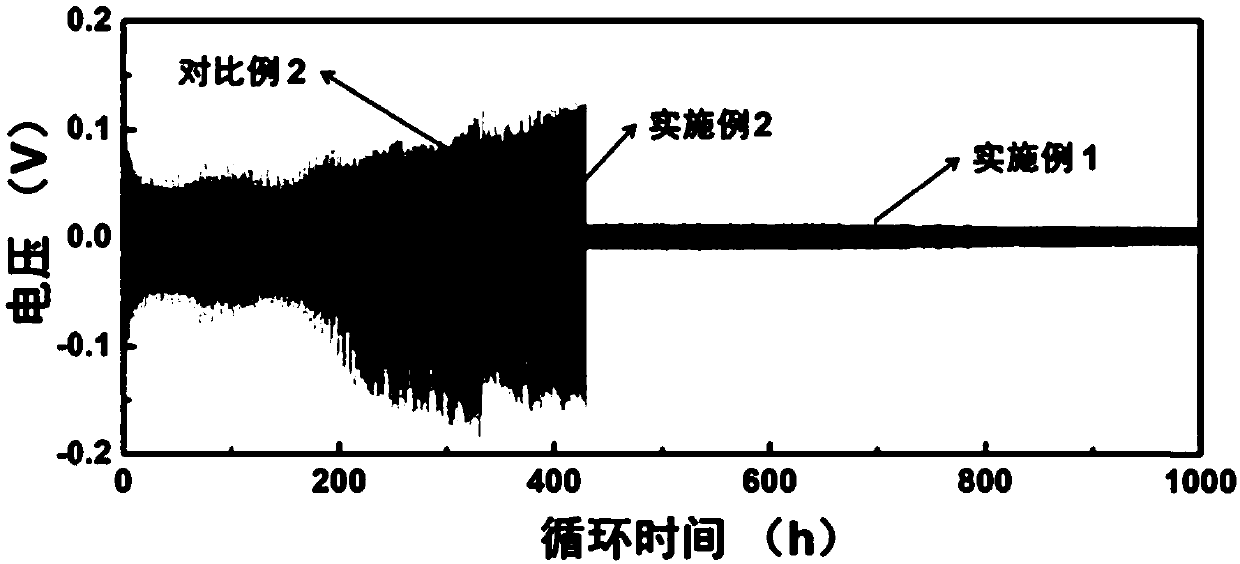
[0038] Using the battery assembly method in Comparative Example 2, it was assembled into a lithium symmetrical button battery, and the AC impedance test was performed, and the battery was tested at 1mA / cm 2 Current density and 1mAh / cm 2 The charge-discharge cycle test is carried out under the deposition and dissolution capacity; assembled into a lithium copper button battery, at 1mA / cm 2 Test the coulombic efficiency and cycle performance of lithium deposition and dissolution under current density conditions; assemble it into a lithium iron phosphate button battery, and test the cycle performance of the battery at a rate of 0.3C.
Embodiment 2
[0040] Lithium discs with a diameter of 16mm (with a surface area of 2cm 2) was uniformly covered with 0.6 g of lithium bisfluorosulfonate imide, and then 0.1 g of DOL was added dropwise to form a polymer electrolyte layer with a thickness of 15 μm. The thickness of the solid lithium salt layer is 15 μm
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com