Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid
A technology of difluoromethyl and methyl groups, applied in the field of preparation of 3--1-methyl-1H-pyrazole-4-carboxylic acid, can solve problems such as spending a lot of energy, and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
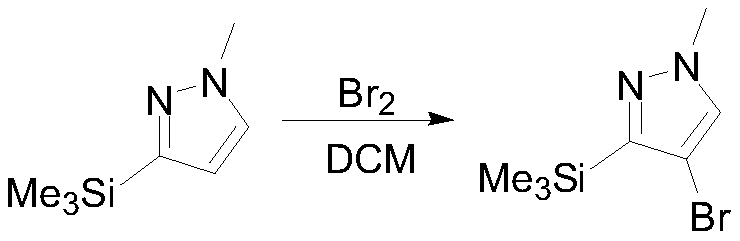
[0030] Under nitrogen protection, 154.3 g (1 mol) of 1-methyl-3-(trimethylsilyl) pyrazole was dissolved in 750 mL of dichloromethane. Cool down to -5-0°C, slowly add 159 g (1 mol) of bromine dropwise, and keep warm to continue the reaction overnight. TLC detects that the reaction is over, add 500mL of water to quench, add dropwise 30% sodium hydroxide aqueous solution to quench the reaction, and adjust the pH=9-10, then add 80mL of saturated aqueous sodium thiosulfate solution, let stand to separate layers, and concentrate the organic phase. Add 300 mL of n-heptane to obtain 208.3 g of 4-bromo-1-methyl-3-(trimethylsilyl)pyrazole, GC: 95.9%, yield 89.3%. 1 HNMR (400MHz, CDCl3) δ: 7.44(s, 1H), 3.95(s, 3H), 0.29(s, 9H).
Embodiment 2
[0032]
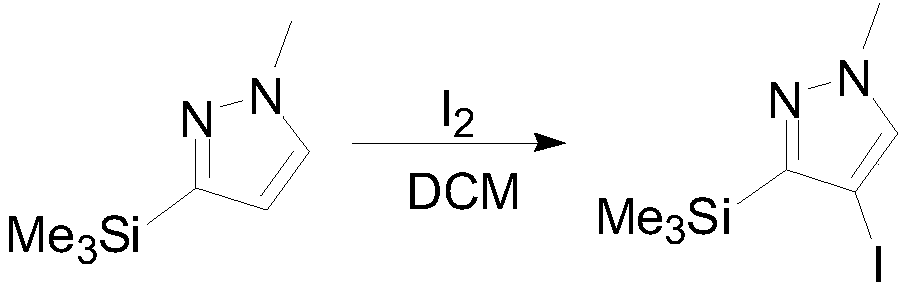
[0033] Under nitrogen protection, 154.3 g (1 mol) of 1-methyl-3-(trimethylsilyl) pyrazole was dissolved in 600 mL of dichloromethane. The temperature was lowered to -50°C, and 251.2 g (1 mol) of iodine was added in batches. After the addition was complete, the reaction was incubated for 5 hours. TLC detects that the reaction is over, adding 500mL of water to quench, standing for stratification, adding saturated aqueous sodium bisulfite to the organic phase for washing, concentrating the organic phase, adding 300mL of n-heptane to obtain 4-iodo-1-methyl-3- (Trimethylsilyl)pyrazole 238.4g, GC: 94.9%, yield 85.1%. 1 HNMR (400MHz, CDCl3): 7.47(s, 1H), 3.97(s, 3H), 0.30(s, 9H).
Embodiment 3
[0035]
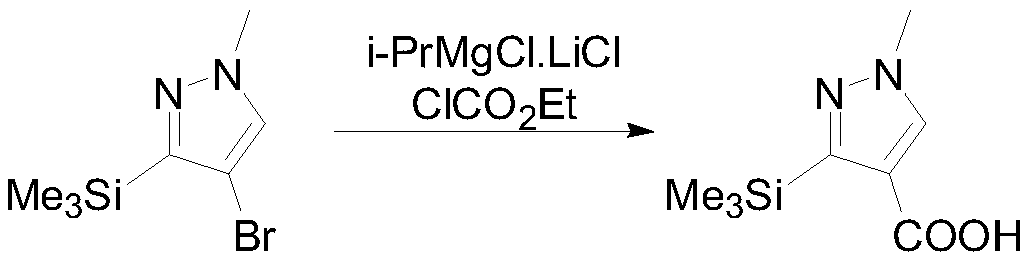
[0036] Under nitrogen protection, 200 g (0.858 mol) of 4-bromo-1-methyl-3-(trimethylsilyl) pyrazole was dissolved in 600 mL 2-methyltetrahydrofuran. Cool down to -5°C, control the temperature range at -5-5°C and slowly add 726mL of 1.3M isopropylmagnesium chloride-lithium chloride in 2-methyltetrahydrofuran solution dropwise, and then raise the temperature to 5-10°C to react for 30 minutes , lower the temperature to -35°C, add 139.6 g of ethyl chloroformate dropwise, react at -35°C to -25°C for 1 hour after the dropwise addition, slowly raise the temperature to 10°C, add saturated ammonium chloride aqueous solution to quench, and separate layers. Extract the water layer with 400mL of 2-methyltetrahydrofuran, combine the organic phases, heat up to 35°C, add 1.25eq of 20% sodium hydroxide aqueous solution dropwise for hydrolysis, TLC detects that the hydrolysis is complete, let stand to separate the layers, keep the water phase, and cool down the water phase to 0°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com