Benzodiazepine compound topical pharmaceutical composition as well as preparation method and application thereof
A composition and compound technology, which is applied in the directions of drug combinations, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve the problems of poor compliance, inconvenient use of preparations for injection, and high production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
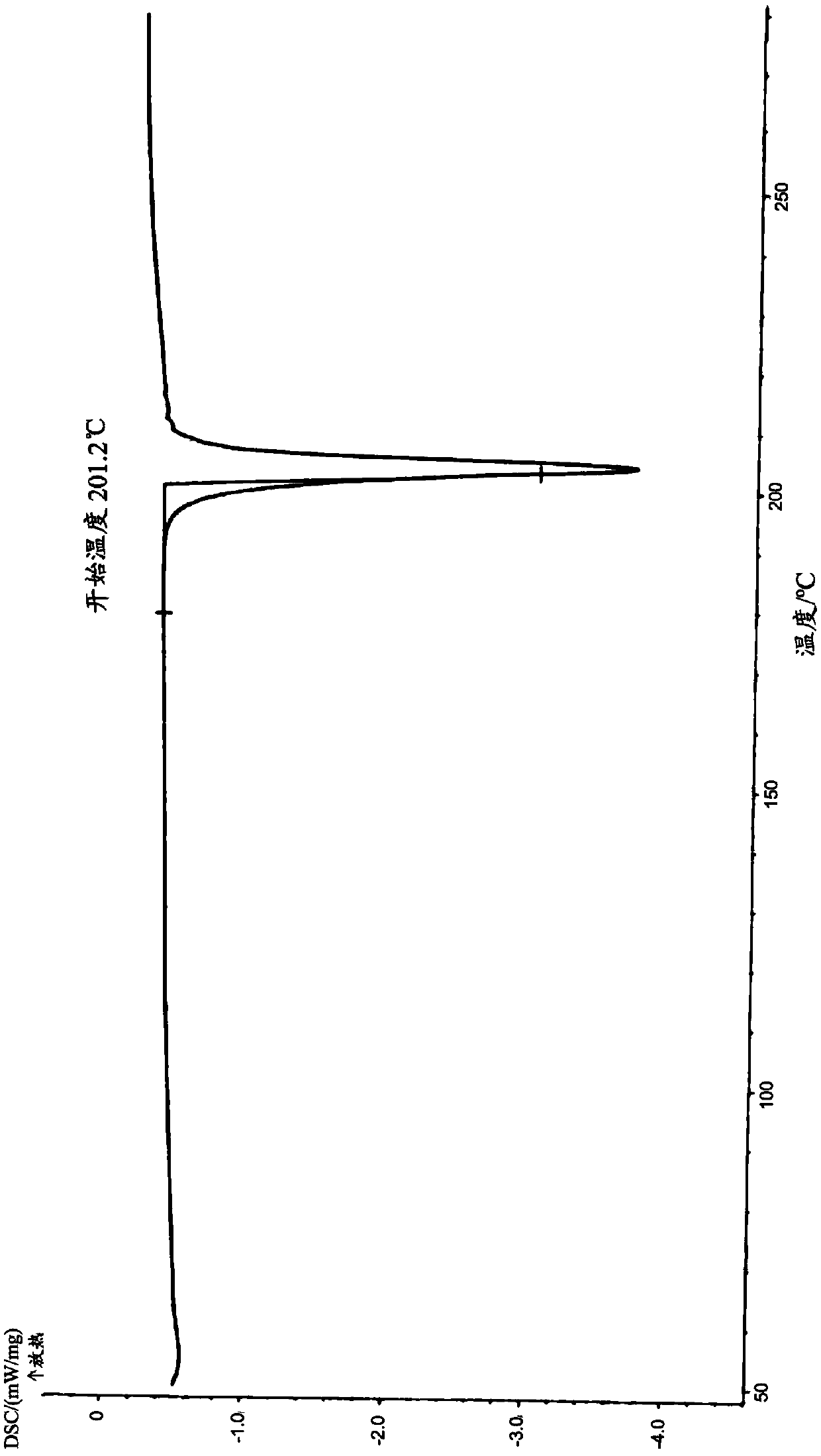
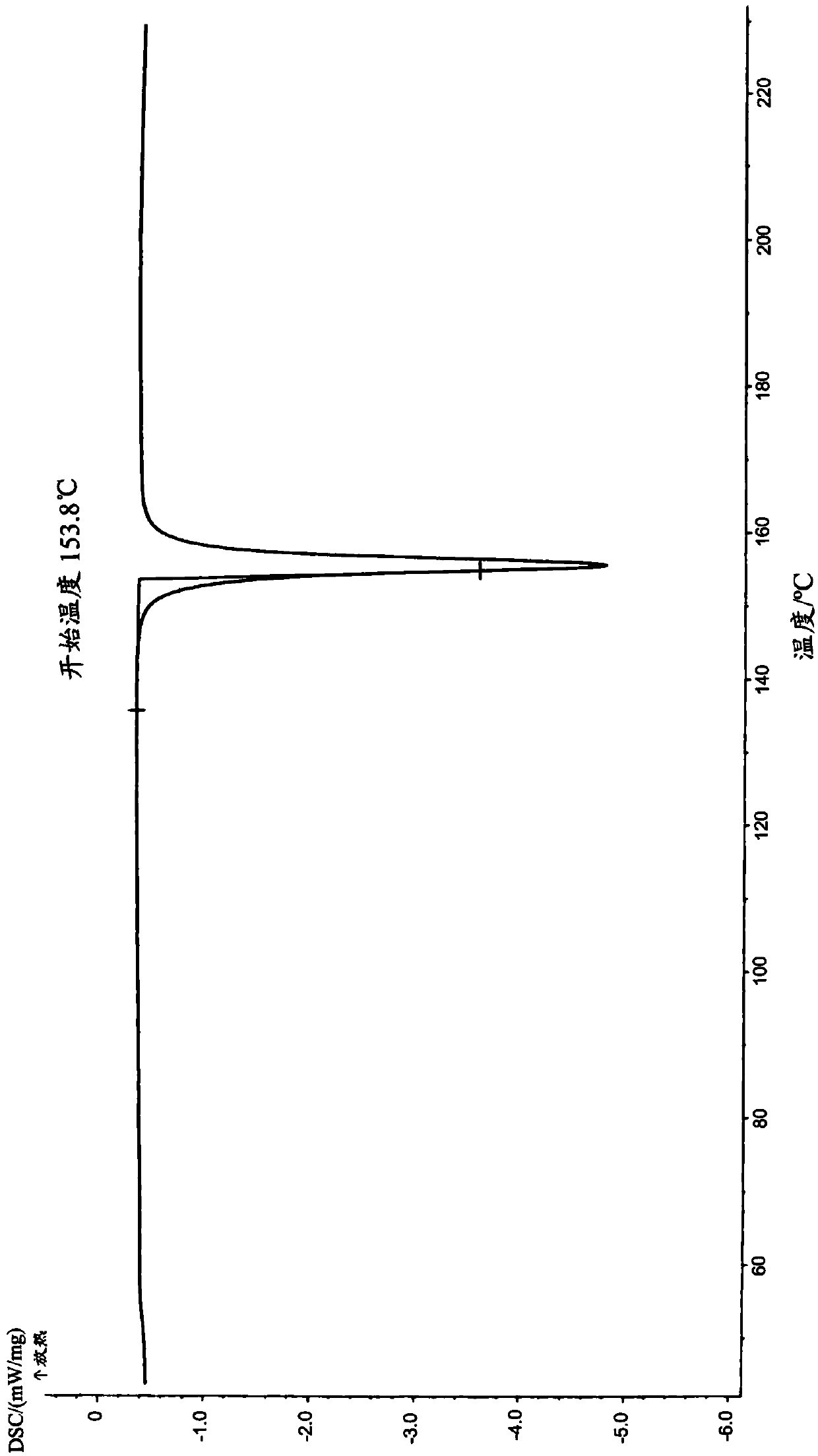
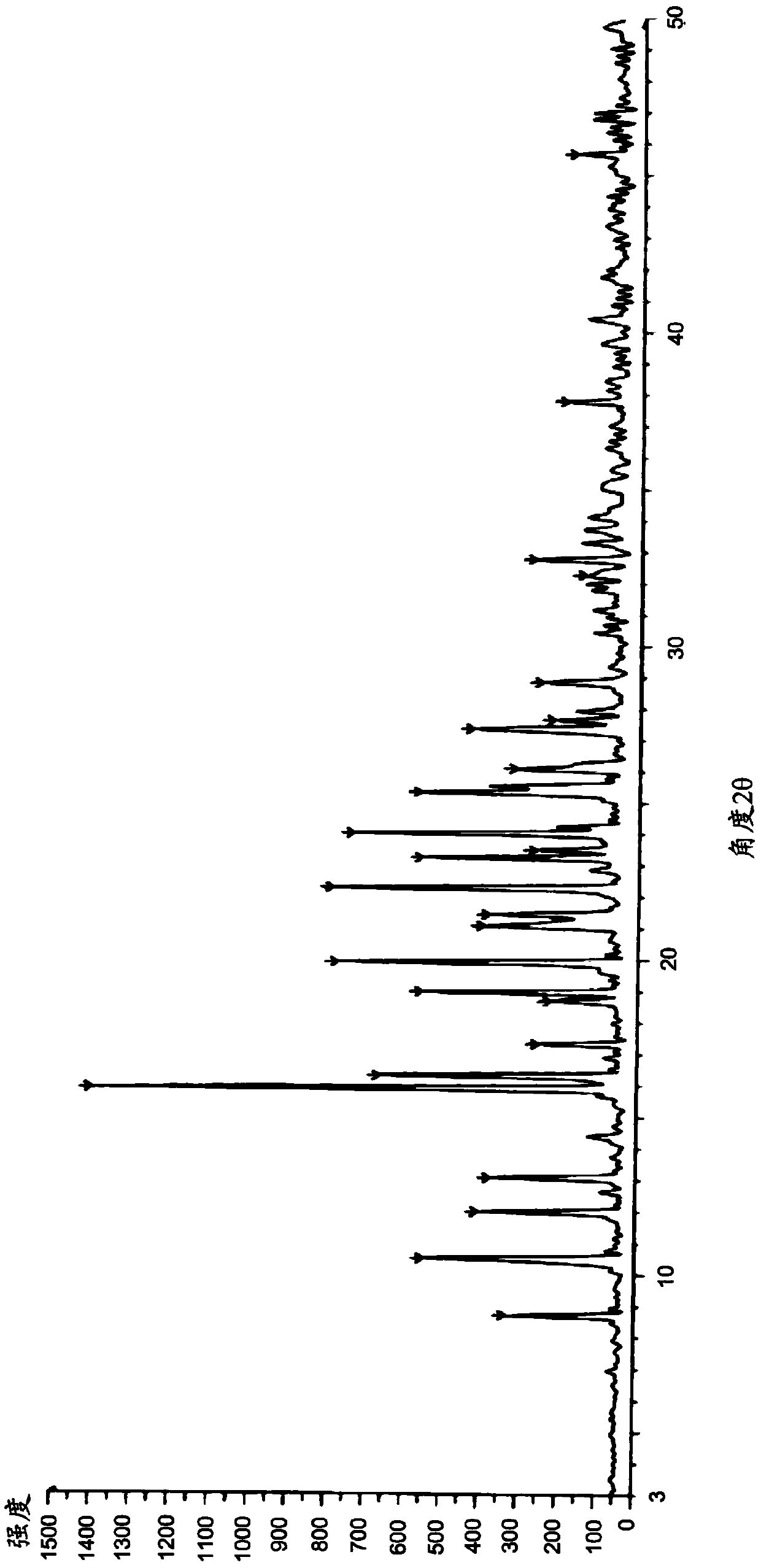
Image
Examples
Embodiment 1
[0075] Example 1 Compound (R) represented by formula (I) 1 is methyl, R 2 Preparation of transdermal patch for methyl)benzenesulfonate
[0076] (1) Compound (R) represented by formula (I) 1 is methyl, R 2 Be methyl) benzene sulfonate 8g and Soluplus7.4g, magnesium stearate 0.5g micronization, add polyethylene glycol (molecular weight 2000) 14g, mix well, make physical mixture;
[0077] (2) Set the extrusion temperature of the twin-screw extruder to be 120°C, start the screw after rising to the set temperature, add the physical mixture in the step (1) to the extruder, and then heat-melt and extrude, It is extruded into spherical particles to obtain amorphous particles, which are then micronized to obtain micronized amorphous particles, and the particle size is controlled at about 100-150 nm.
[0078] (3) Weigh 3 g of micronized amorphous particles prepared in step (2), 2 g of soybean lecithin, 0.3 g of cholesterol, 30 g of dehydrated alcohol, and 64.7 g of water;
[0079] ...
Embodiment 2
[0086] Embodiment 2 Compound (R) represented by formula (I) 1 is methyl, R 2 Preparation of transdermal patch for ethyl)
[0087] (1) Compound (R) represented by formula (I) 1 is methyl, R 2 For ethyl) 0.9g and povidone (PVP-S630) 3.1g, talc 0.2g micronized, add polyethylene glycol (molecular weight 4000) 5.8g, mix uniformly to obtain a physical mixture;
[0088] (2) Set the extrusion temperature of the twin-screw extruder to be 120°C, start the screw after rising to the set temperature, add the physical mixture in the step (1) to the extruder, and then heat-melt and extrude, It is extruded into spherical particles to obtain amorphous particles, which are then micronized to obtain micronized amorphous particles, and the particle size is controlled at about 150-175 nm.
[0089] (3) Weighing the compound (R) represented by the formula (I) prepared in step (2) 1 is methyl, R 2 ethyl) micronized amorphous particles 0.3g, phosphatidylcholine 2g, cholesterol 0.3g, propylene gl...
Embodiment 3
[0097] Embodiment 3 Compound (R) represented by formula (I) 1 for hydrogen, R 2 Preparation of transdermal patch for methyl)
[0098] (1) Compound (R) represented by formula (I) 1 for hydrogen, R 2 Be methyl) 0.8g and povidone (PVP-K30) 3g, magnesium stearate 0.2g micronize, add polyethylene glycol (molecular weight 6000) 6.0g, mix well, make physical mixture;
[0099] (2) The extrusion temperature of the twin-screw extruder is set to 120°C, the screw is started after rising to the set temperature, the physical mixture in step (1) is added to the extruder, and after hot melting and extrusion, It is extruded into spherical particles to obtain amorphous particles, which are then micronized to obtain micronized amorphous particles, and the particle size is controlled at about 180-230 nm.
[0100] (3) Weighing the compound (R) represented by the formula (I) prepared in step (2) 1 for hydrogen, R 2 For methyl) micronized amorphous particles 7g, phosphatidylethanolamine 2g, ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com