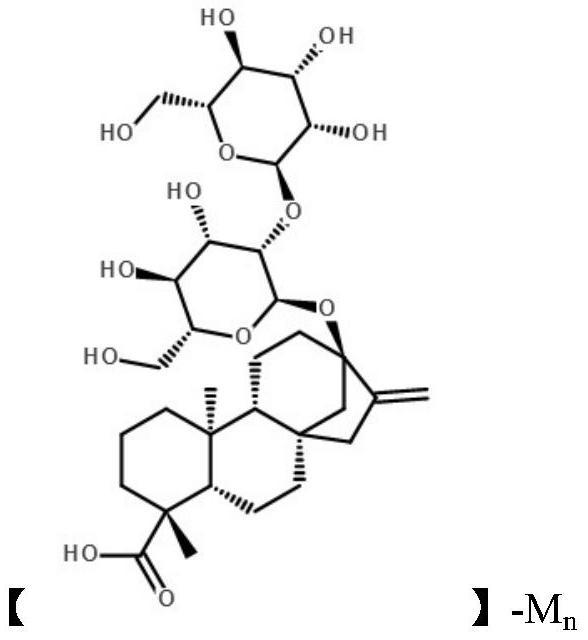
A kind of stevioside metal copper complex and preparation method thereof
A technology of steviolbioside and metal copper, which is applied in the field of preparation of metal complexes, and achieves the effects of strong operability, excellent DPPH removal and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
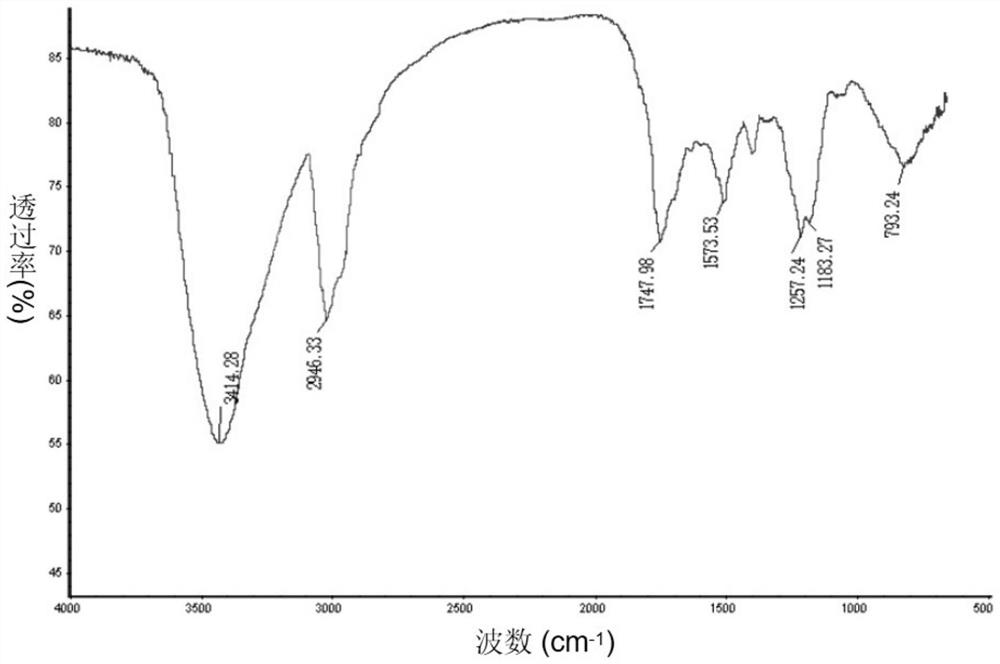
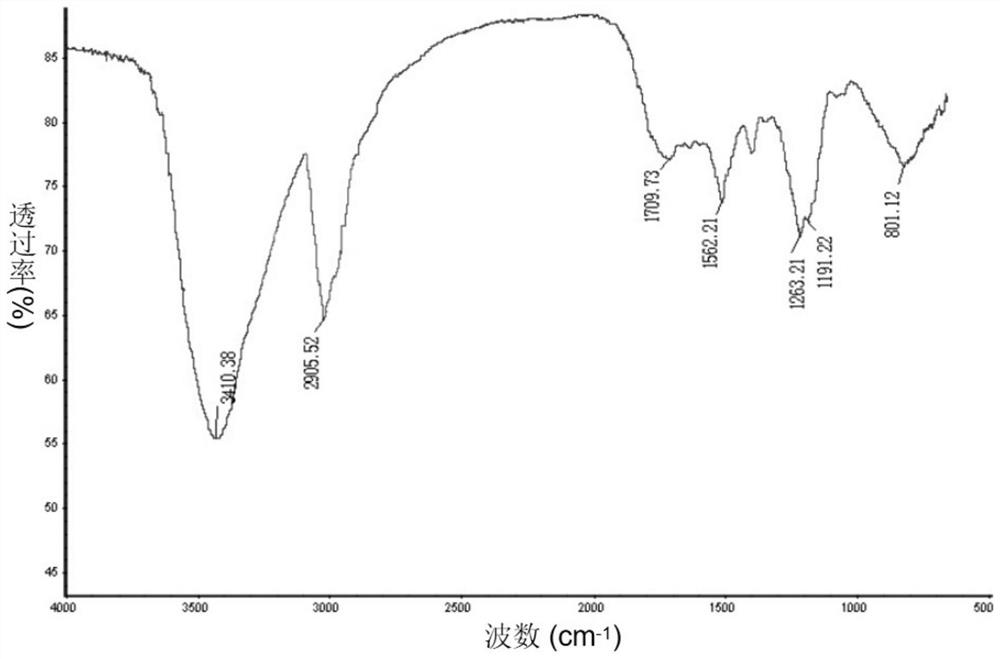
Image
Examples
Embodiment 1
[0060] 1. Dissolution: fully dissolve 100 g of steviolbioside with a content of 85.2 wt% in 400 ml of 95% ethanol, dissolve 15 g of basic copper sulfate in 120 ml of an apple aqueous solution with a pH of 6.0, and fully stir to dissolve.
[0061] 2. Reaction: Mix the two solutions in step 1 and adjust the pH to 8.0 with ammonia water, then add 0.3 g of triethanolamine with stirring and react at a constant temperature of 60°C for 8 hours.
[0062] 3. Crystallization: Cool the reaction solution in step 2 to 10°C, let the crystal continue to grow, and keep it for 24 hours, then separate the crystal from solid and liquid by suction filtration, and use 90% ethanol at -4°C Rinse the extract until the eluate is colorless.
[0063] 4. Drying: Place the obtained crystals in a blast drying oven at 55°C for pre-drying until there is no obvious alcohol smell, then raise the temperature to 80°C and continue drying to constant weight. Promptly obtain 89.6g steviolbioside metal copper compl...
Embodiment 2
[0065] 1. Dissolution: fully dissolve 100g of 85.twt% steviolbioside in 600ml of 90% methanol, dissolve 15g of basic copper chloride in 150ml of citric acid solution with a pH of 6.0, and fully stir to dissolve.
[0066] 2. Reaction: Mix the two solutions in step 1 and adjust the pH to 8.0 with ammonia water, then add 0.4 g of tripropylamine, and react with stirring at a constant temperature of 80°C for 10 hours.
[0067] 3. Crystallization: Cool the reaction liquid in step 2 to -4°C, let the crystal continue to grow, and keep it for 12 hours, then separate the crystal from solid and liquid by suction filtration, and use 90% of the -4°C Methanol was used to rinse the leaching until the eluent was colorless.
[0068] 4. Drying: Place the obtained crystals in a vacuum drying oven with a vacuum degree of -0.8Mpa and a temperature of 45°C for pre-drying until there is no obvious alcohol smell, and then raise the temperature to 60°C and continue drying to constant weight. That is,...
Embodiment 3
[0070] 1. Dissolution: Fully dissolve 100g of steviolbioside with a content of 85.2wt% in 800ml of 90% isopropanol, dissolve 15g of basic copper chloride in 150ml of a citric acid solution with a pH of 6.0, and stir well to dissolve .
[0071] 2. Reaction: Mix the two solutions in step 1 and adjust the pH to 8.0 with ammonia water, then add 0.3 g of 4-aminotoluene-3-sulfonic acid, and react with stirring at a constant temperature of 75° C. for 10 h.
[0072] 3. Crystallization: Cool the reaction solution in step 2 to 0°C, let the crystal continue to grow, and keep it for 16 hours, then separate the crystal from solid and liquid by suction filtration, and use 90% isopropyl alcohol at 0°C Alcohol was used to rinse the extract until the eluate was colorless.
[0073] 4. Drying: Place the obtained crystals in a vacuum oven with a vacuum degree of -0.1Mpa and a temperature of 40°C for pre-drying until there is no obvious alcohol smell, and then raise the temperature to 80°C and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com