Preparation method of andrographolide enteric-coated tablet
A technology of andrographolide and enteric-coated tablets, which is applied in the field of medicine, can solve problems such as easy degradation, and achieve the effects of improving bioavailability, taking convenience, and ensuring curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
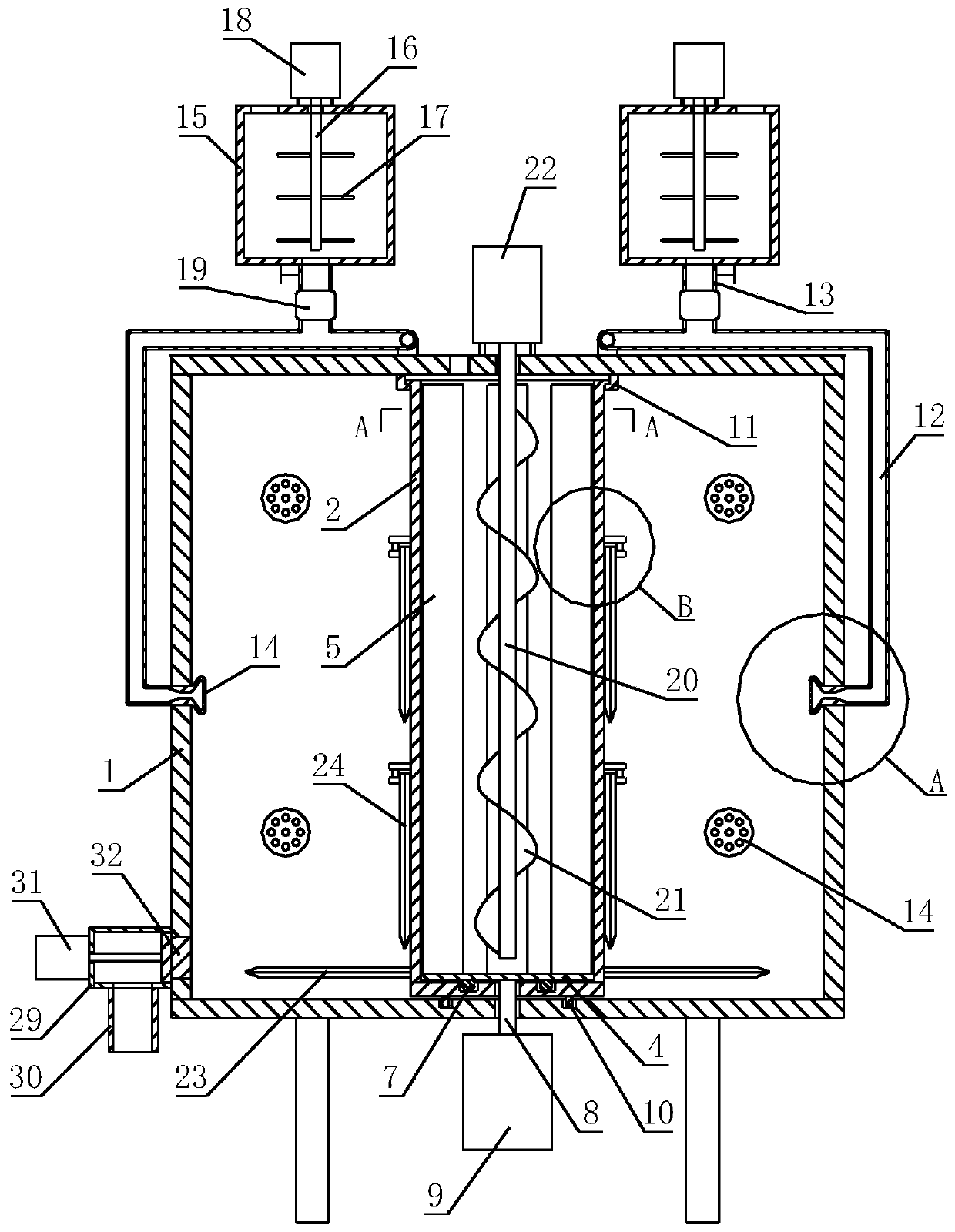
[0041] The invention discloses a preparation method of andrographolide enteric-coated tablets, which specifically comprises the following steps:
[0042] (1) Preparation of tablet core
[0043] Dissolve hydroxypropyl cellulose with 20% to 95% ethanol aqueous solution, and then add sodium lauryl sulfate to prepare a solution with a solid content of 3% to 15% as a binder;
[0044] After sieving andrographolide, lactose and microcrystalline cellulose, add them to the high-speed mixing wet granulation device and mix them, then add the prepared binder for granulation; then add the prepared drug granules to the boiling dryer for drying , the water content is controlled at 2% to 5%; then the dried drug granules are sized with a granulator, and finally compressed with a high-speed rotary tablet machine to obtain the core of andrographolide enteric-coated tablets; andrographolide , Lactose and microcrystalline cellulose are routinely selected according to the actual situation.
[004...
Embodiment
[0064] The above method was used to prepare andrographolide enteric-coated tablets, and the specific composition ratios are shown in Table 1 below.
[0065] Table 1 Raw material ratio (parts by weight)
[0066]
[0067] Tablet cores were prepared according to the proportions in Table 1. The parameters during the operation of the high-speed mixing wet granulation device are shown in Table 2 below. The proportions of each group in the above Table 1 were prepared using the parameters set in Table 2 below. .
[0068] Table 2 Concrete Process Parameters
[0069]
[0070] After the preparation of the tablet core is completed, the tablet core is coated with an isolation layer and an enteric-coated layer, and the process parameters of the coating machine during the process of coating the isolation layer and the enteric-coated layer are shown in Table 3 below.
[0071] The process parameter of table 3 coating machine
[0072]
[0073] After each group of raw materials in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com