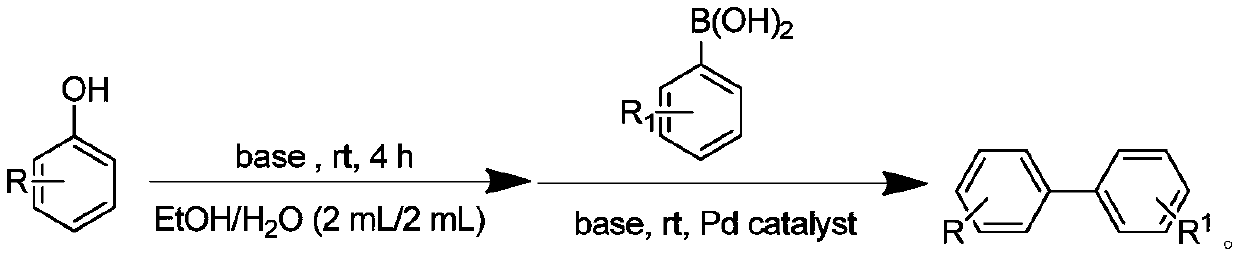
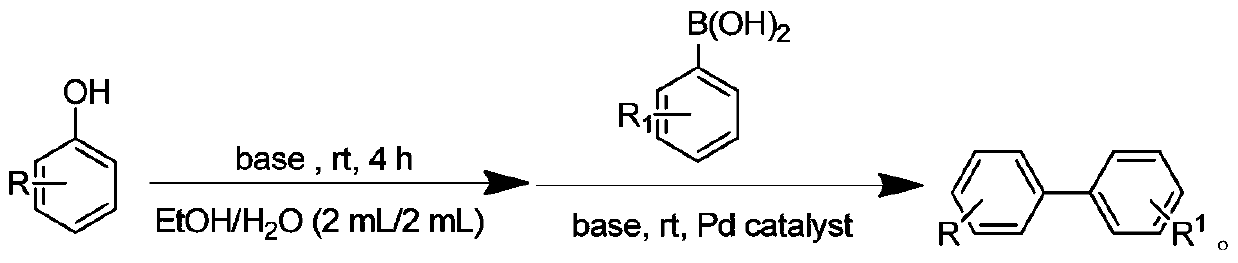
Method for synthesizing biphenyl compound by taking phenol as raw material
A compound and phenol technology, applied in the field of synthesizing biphenyl compounds, can solve the problems of toxic reagents and expensive raw materials, and achieve the effects of simplified operation, low price and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the preparation of 4-nitrobiphenyl
[0019] In the air, sequentially add 1mmol of 4-nitrophenol, 3mmol of triethylamine, and 4mL of 50% ethanol aqueous solution into a 10mL flask, slowly introduce sulfonyl fluoride gas, and magnetically stir the reaction at room temperature. After 4 hours of reaction, Add 1mmol of phenylboronic acid, 3mmol of triethylamine, 0.1mmol of palladium acetate to the reaction, and continue to react at room temperature for 6 hours. After the reaction is completed, add 20mL of saturated saline to quench the reaction, and the reaction mixture is extracted with 40mL of ethyl acetate. The organic phase and the filtrate were concentrated, and the concentrated filtrate was separated by column chromatography to obtain the final product. The structure of the product was identified by proton nuclear magnetic resonance and mass spectrometry, and the separation yield reached 94%.
[0020] 1 H NMR (400MHz, CDCl 3 )δ8.30(d, J=8.9Hz, 2H), 7.74...
Embodiment 2
[0021] Embodiment 2: the preparation of 4-aminobiphenyl
[0022] In the air, sequentially add 1mmol of 4-aminophenol, 3mmol of triethylamine, and 4mL of 50% ethanol aqueous solution into a 10mL flask, slowly introduce sulfonyl fluoride gas, and magnetically stir the reaction at room temperature. After 4 hours of reaction, the reaction Add 1mmol of phenylboronic acid, 3mmol of triethylamine, 0.2mmol of palladium acetate, and continue to react at room temperature for 6 hours. After the reaction is completed, add 20mL of saturated saline to quench the reaction. The reaction mixture is extracted with 40mL of ethyl acetate. phase, the filtrate was concentrated, and separated by column chromatography to obtain the final product. The structure of the product was identified by proton nuclear magnetic resonance and mass spectrometry, and the separation yield reached 91%. 1 HNMR (400MHz, CDCl 3 )δ7.53(d, J=6.9Hz, 2H), 7.41(t, J=9.5Hz, 4H), 7.34–7.06(m, 1H), 6.75(d, J=7.2Hz, 2H), 3.71( ...
Embodiment 3
[0023] Embodiment 3: the preparation of 3-aminobiphenyl
[0024] In the air, sequentially add 1mmol of 3-aminophenol, 3mmol of triethylamine, and 4mL of 50% ethanol aqueous solution into a 10mL flask, slowly introduce sulfonyl fluoride gas, and magnetically stir the reaction at room temperature. After 4 hours of reaction, the reaction Add 1mmol of phenylboronic acid, 3mmol of triethylamine, 0.2mmol of palladium acetate, and continue to react at room temperature for 6 hours. After the reaction is completed, add 20mL of saturated saline to quench the reaction. The reaction mixture is extracted with 40mL of ethyl acetate. phase, the filtrate was concentrated, and separated by column chromatography to obtain the final product. The structure of the product was identified by proton nuclear magnetic resonance and mass spectrometry, and the separation yield reached 91%.
[0025] 1 H NMR (400MHz, CDCl 3 )δ7.55(d, J=7.1Hz, 2H), 7.40(t, J=7.5Hz, 2H), 7.31(t, J=7.3Hz, 1H), 7.20(t, J=7.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com