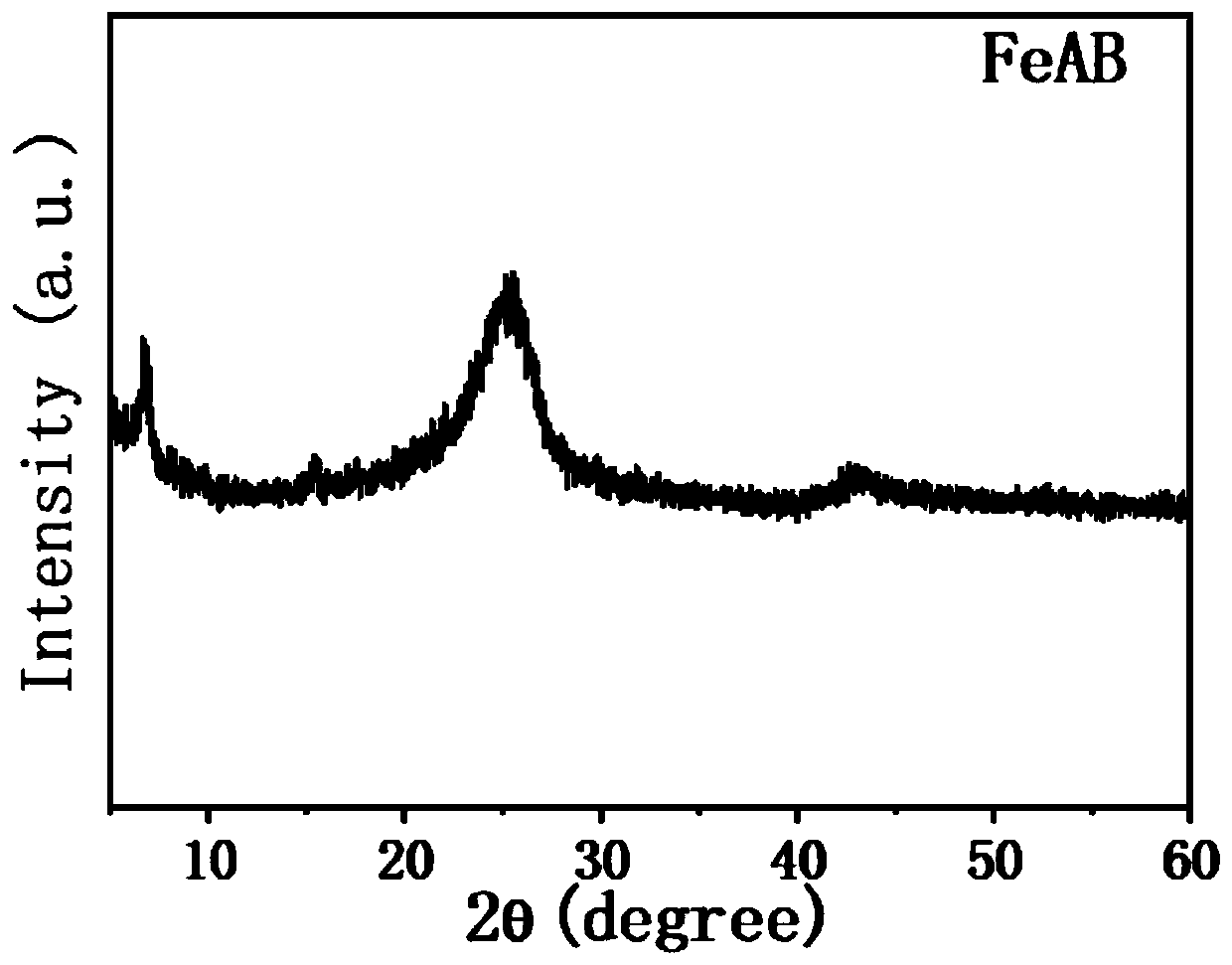
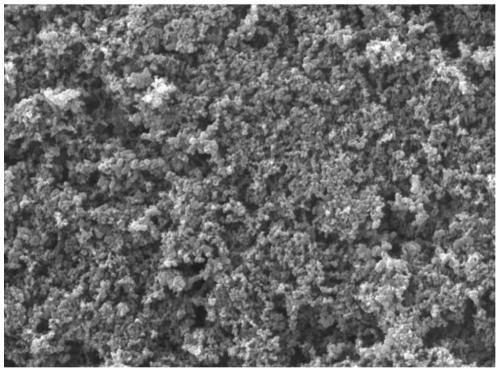
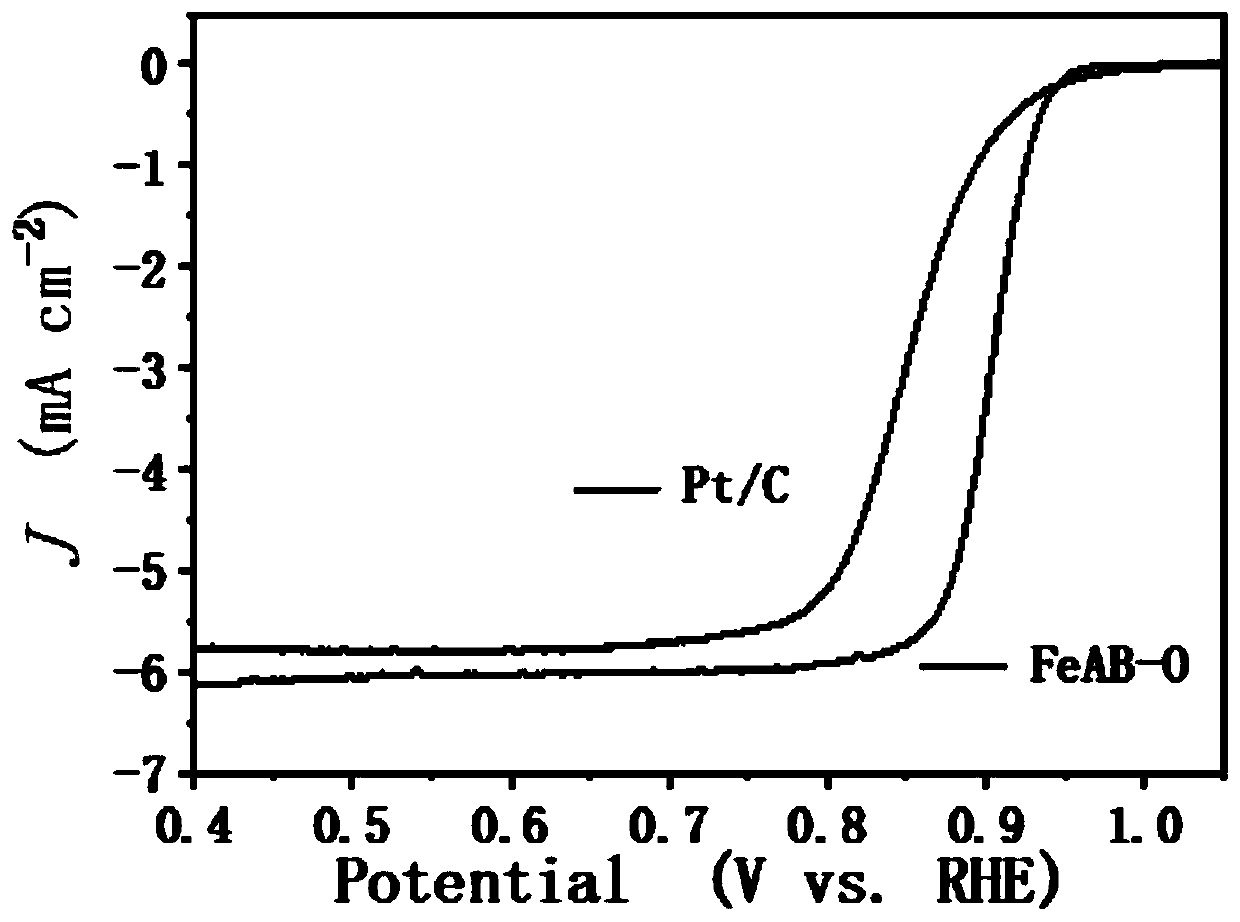
Preparation method of high-activity electrochemical oxygen reduction catalyst
A catalyst and electrochemical technology, applied in the field of preparation of high-activity electrochemical oxygen reduction catalysts, can solve the problems of hindering electrochemical oxygen reduction efficiency, poor electrical conductivity of iron phthalocyanine, unable to maintain stability, etc., and achieve high electrochemical oxygen reduction. Effects of reduction efficiency, low cost of raw materials, and good electrochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In this example, first take 5 mg of iron phthalocyanine and add it to 80 ml of N,N-dimethylformamide, ultrasonic for 1 hour at a speed of 500 r min -1 , stirred for 30 minutes to completely dissolve the iron phthalocyanine, so that there is no precipitation in the organic solvent; then take 35 mg of acetylene black bombarded by oxygen plasma (process parameters are as follows: power 90W, time 40min) and add it, keep it sealed, and rotate at a speed of 500r min -1 , stirred for 35 hours, reacted fully, let the phthalocyanine material adsorb on the conductive material, separated the solid black substance in the solution by suction filtration, washed it with deionized water and ethanol for several times, and opened it under the condition of 60°C After standing for 9 hours to dry, a black solid powder catalyst was obtained, that is, the highly active electrochemical oxygen reduction catalyst. In this embodiment, in terms of mass percentage, the content of phthalocyanine in ...
Embodiment 2
[0034] In this example, first take 8 mg of iron phthalocyanine and add it to 90 ml of N,N-dimethylformamide, ultrasonic for 1 hour at a speed of 500 r min -1 , stirred for 1h, iron phthalocyanine was completely dissolved, and there was no precipitation in the organic solvent; then 40mg of Ketjen black was added after oxygen plasma bombardment (process parameters were as follows: power 100W, time 35min), kept sealed, and the speed was 500r. min -1, stirred for 30 hours, fully reacted, let the phthalocyanine material adsorb on the conductive material, separated the solid black substance in the solution by suction filtration, washed it with deionized water and ethanol for several times, and opened it under the condition of 50 ℃ After standing for 10 h to dry, a black solid powder catalyst was obtained, that is, the highly active electrochemical oxygen reduction catalyst. In this embodiment, in terms of mass percentage, the content of phthalocyanine in the composition of the blac...
Embodiment 3
[0036] In this example, first take 5 mg of iron phthalocyanine and add it to 80 ml of N,N-dimethylformamide, ultrasonic for 1 hour at a speed of 500 r min -1 , stirred for 1h to completely dissolve the iron phthalocyanine, so that there is no precipitation in the organic solvent; then take 40mg of graphene bombarded by oxygen plasma (process parameters are as follows: power 70W, time 30min) and add it, keep it sealed, and rotate at a speed of 500r min -1 , stirred for 40 hours, fully reacted, let the phthalocyanine material adsorb on the conductive material, separated the solid black substance in the solution by suction filtration, washed it with deionized water and ethanol for several times, and opened it under the condition of 70 ℃ After standing for 8 hours to dry, a black solid powder catalyst was obtained, that is, the highly active electrochemical oxygen reduction catalyst. In this embodiment, in terms of mass percentage, the content of phthalocyanine in the composition ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com