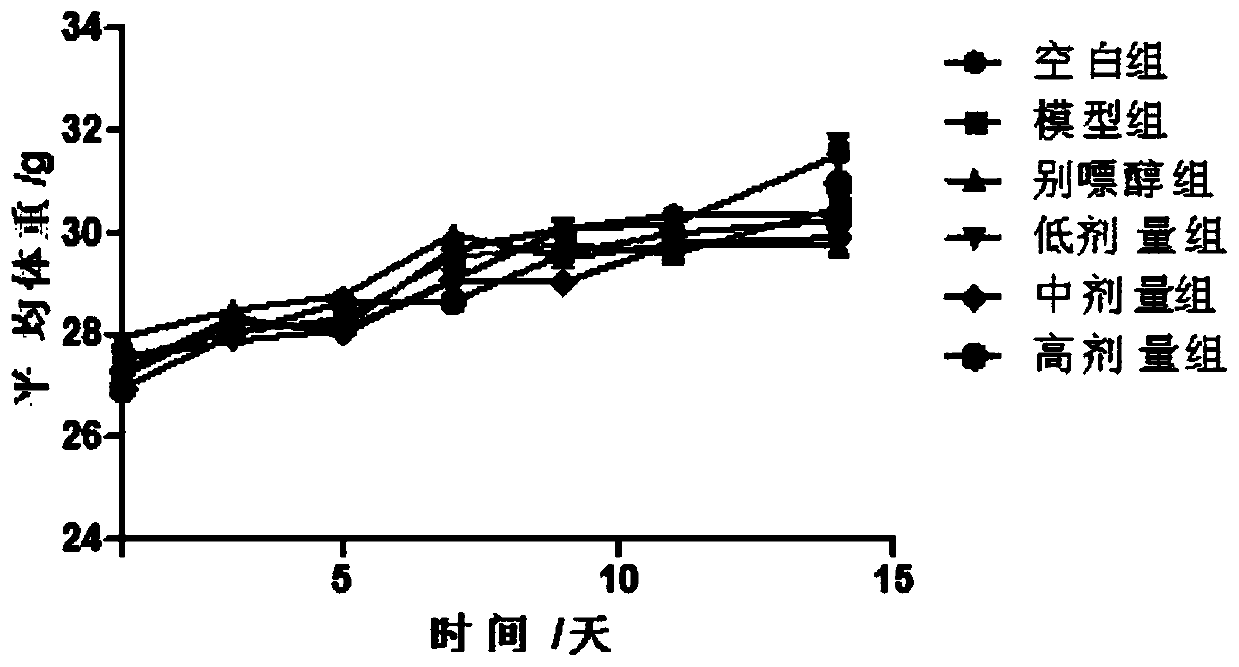
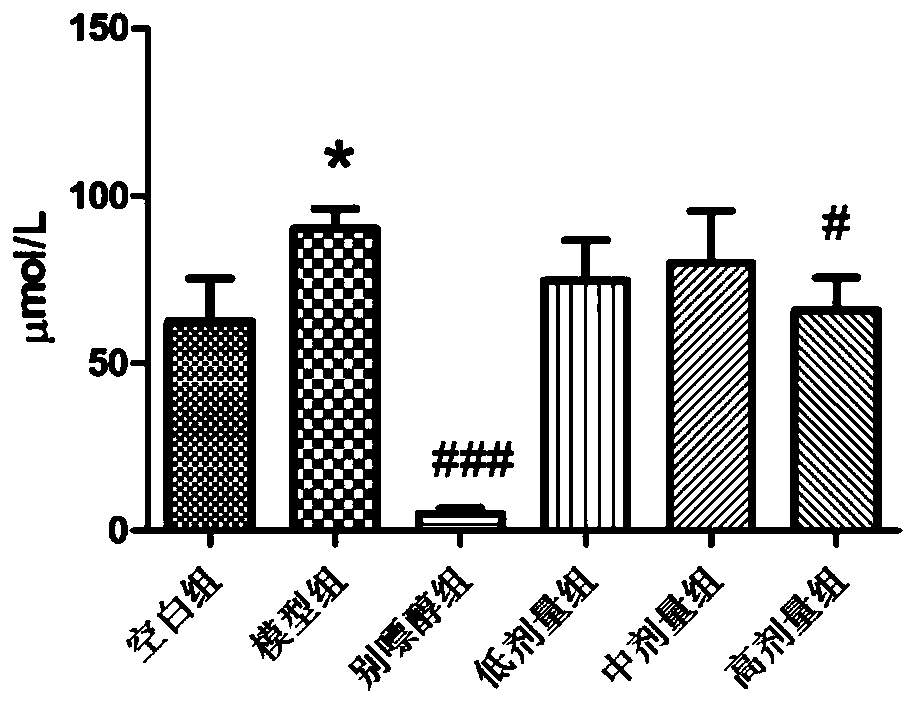
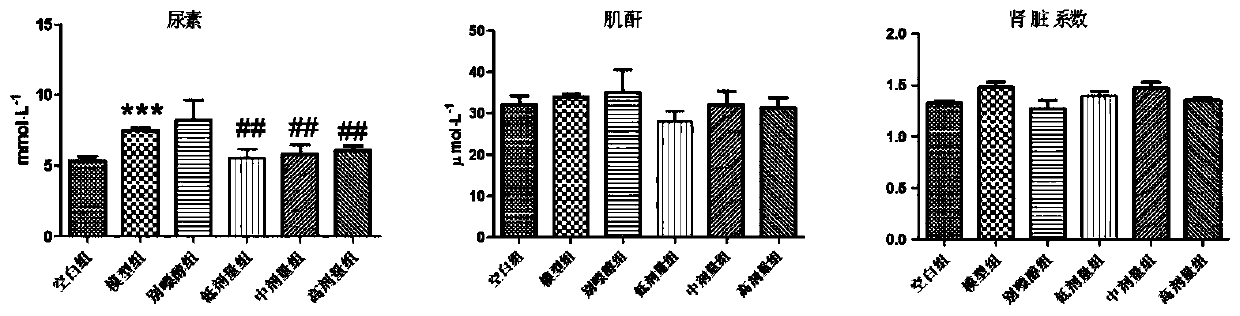
Pharmaceutical composition for treating hyperuricemia and preparation method and use of pharmaceutical composition
A technology of hyperuricemia and composition, applied in the field of medicine, which can solve the problems of complicated etiology and disease, difficulty in controlling dosage, insufficient medicine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: the preparation of the pharmaceutical composition granule for the treatment of hyperuricemia
[0083] Take 700kg of Qinpi and 500kg of Clematis, soak them in water, add 7-12 times the amount of water to decoct 3 times, each decocting time is 40-100 minutes (collect volatile oil during each decoction), and decoct together Liquid, filtered, concentrated to a relative density of 1.08-1.12, spray-dried to make extract powder, sieved, added dextrin and mixed evenly, and then added sweet substitute sugar and mixed evenly. Add 92% ethanol, sieve, dry at 40-60°C, granulate after sieving, spray into volatile oil, seal and moisten for 65-70 minutes, pack separately, and obtain the pharmaceutical composition granules for treating hyperuricemia.
Embodiment 2
[0084] Embodiment 2: the preparation of the pharmaceutical composition oral liquid for the treatment of hyperuricemia
[0085] Take 700kg of Qinpi and 500kg of Clematis, soak them in water, add 7-12 times the amount of water to decoct 3 times, each decocting time is 40-100 minutes (collect volatile oil during each decoction), and decoct together Liquid, filtered, concentrated to a relative density of 1.08-1.12, added ethanol to make the alcohol content reach 70-80%, let it stand for 24 hours, filtered, and the filtrate was decompressed to recover ethanol and then centrifuged; the supernatant was added to the collected volatile oil and polysorbate Ester, cyclamate and sorbic acid are mixed evenly, added with water, filtered, filled and sterilized to obtain a pharmaceutical composition oral liquid for treating hyperuricemia.
experiment example 3-4
[0086] In the following experimental examples 3-4:
[0087] ①Experimental animals
[0088] Kunming mice (KM), 18-22g, male, SPF grade, provided by Chengdu Dashuo Experimental Animal Co., Ltd.
[0089] SD rats, all male, SPF grade, provided by Chengdu Dashuo Experimental Animal Co., Ltd.
[0090] ②Experimental drugs and reagents
[0091] Potassium oxonate (98%), Shanghai Aladdin Biochemical Technology Co., Ltd. or Shanghai McLean Biochemical Technology Co., Ltd., was prepared with normal saline solution to a concentration of 25 mg / ml before use.
[0092] Yeast extract, Beijing Aobo Biotechnology Co., Ltd., was dissolved in hot water to a solution of 750mg / ml before use.
[0093] Allopurinol, Hefei Jiulian Pharmaceutical Co., Ltd. or Hefei Jiulian Pharmaceutical Co., Ltd., dissolved in hot water to a 2mg / ml solution before use.
[0094] Xanthine oxidase (XOD) test kit, Nanjing Jiancheng Institute of Bioengineering, Jiangsu.
[0095] Total protein quantitative test kit (BCA ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com