Method for preparing sulfuric acid from ferrous sulfate
A technology of ferrous sulfate and sulfuric acid, applied in chemical instruments and methods, iron oxides, sulfur compounds, etc., can solve the problems of low utilization rate of ferrous sulfate and large scale of sulfuric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] FeSO 4 ·7H 2 Experiment of mixed calcination of O and coal in air
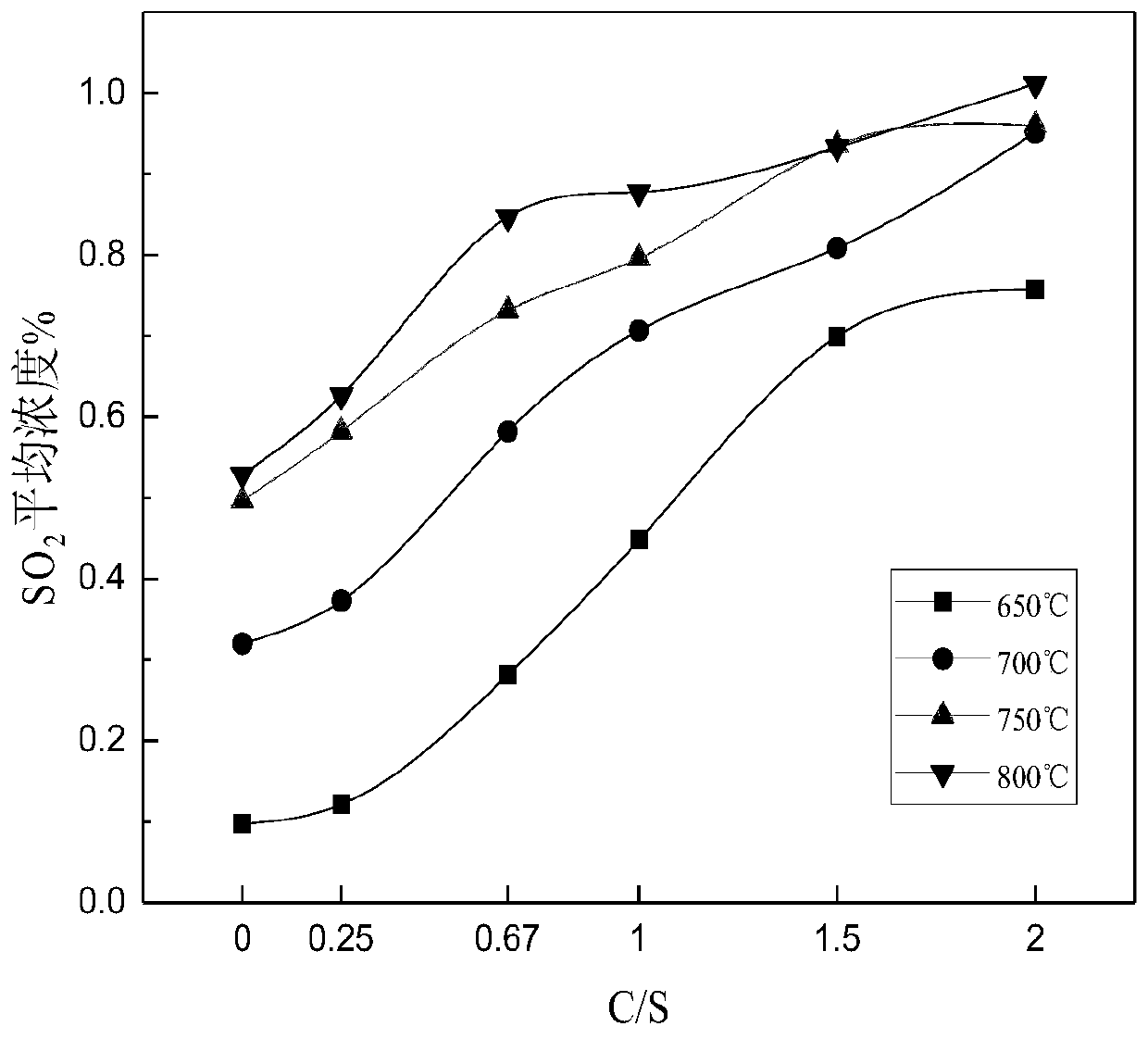
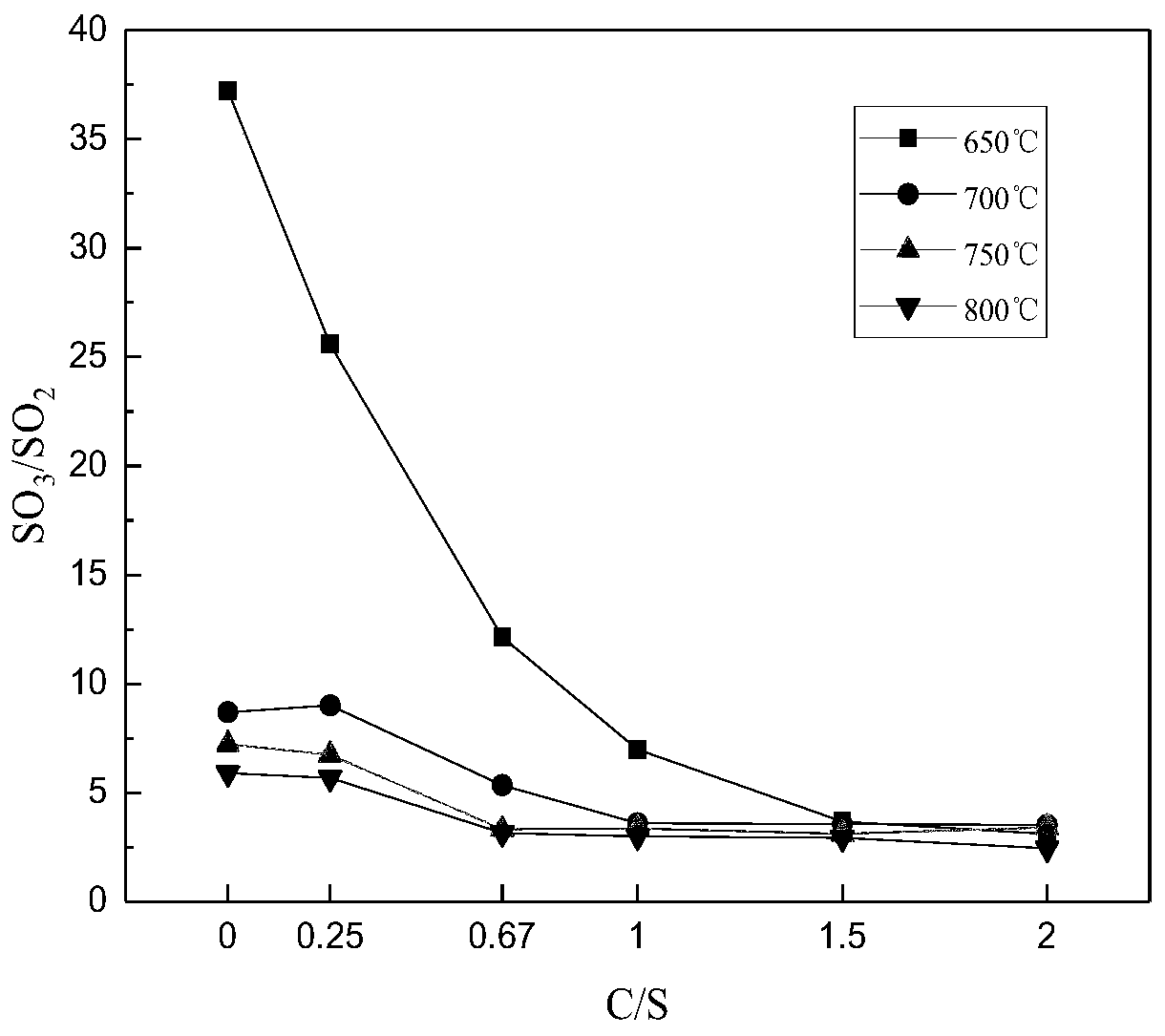
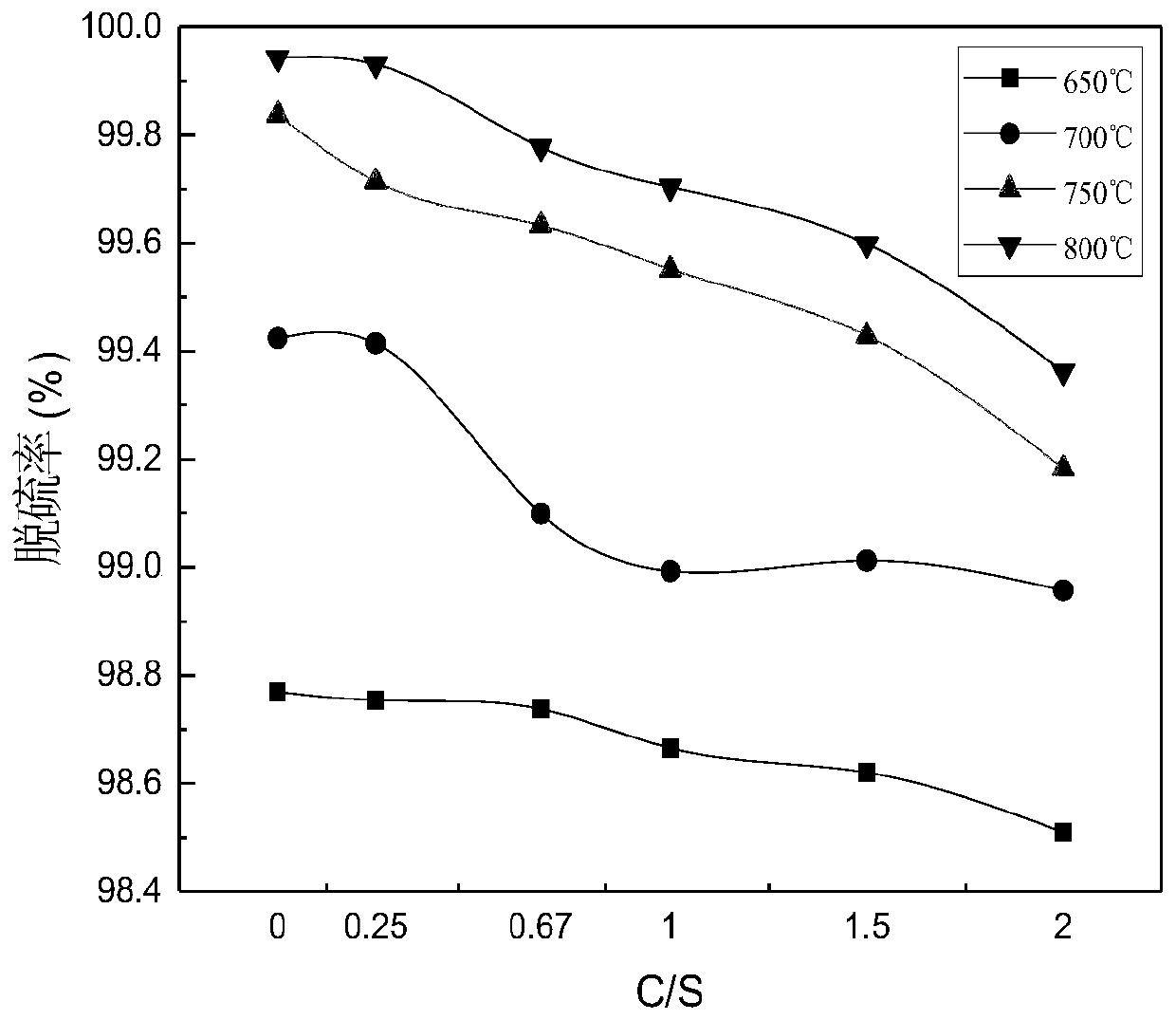
[0075] Analytical pure ferrous sulfate heptahydrate is used as the reaction raw material, coal washing is selected as ferrous sulfate thermal decomposition reducing agent, and analytical pure FeSO 4 ·7H 2 O is mixed with coal at different C / S (molar ratios) and fed into an atmosphere tube furnace for calcination at different temperatures in an air atmosphere. The selection of C / S value is 0.25, 0.67, 1, 1.5, 2 according to the reaction equation of ferrous sulfate and coal. The experimental temperature was selected as 650°C, 700°C, 750°C, and 800°C according to the thermogravimetric results.
[0076] The result is shown in Figure 1.
[0077] Figure 1(a) shows the FeSO 4 ·7H 2 O decomposes to produce SO in the furnace gas 2 It can be seen from Figure 1(a) that under the air atmosphere, a small amount of coal reduces and decomposes ferrous sulfate 2 The concentration is very low. However, after a...
Embodiment 2
[0082] Separate Calcination of Industrial Ferrous Sulfate
[0083] Raw material industrial FeSO 4 ·H 2 O is finely ground and passed through a 100-mesh standard sampling sieve; then, the temperature of the atmosphere tube furnace is raised to the specified temperature, and 2.0g of industrial FeSO is weighed. 4 ·H 2 O is placed in an atmosphere tube furnace and calcined at different temperatures in an air atmosphere, which produces SO 2 The relationship between the instantaneous concentration and time is shown in Figure 2, and the calculation of FeSO 4 ·H 2 O conversion rate and desulfurization rate. The decomposition temperature of some sulfuric acid impurity salts contained in industrial ferrous sulfate is higher than 800°C, so the reaction temperature is selected as 800°C, 850°C, 900°C, and 950°C in the examples.
[0084] Refer to Figure 2 for the results. Figure 2(a) shows the FeSO at different temperatures in the air atmosphere 4 ·H 2 After O is calcined alone, SO...
Embodiment 3
[0086] Oxygen concentration on industrial FeSO 4 4H 2 Effect of O Ferrous Sulfate Decomposition
[0087] Raw material industrial FeSO 4 4H 2 O and coal are finely ground and passed through a 100-mesh standard sieve; the reducing agent coal and industrial FeSO 4 4H 2 O was mixed according to different C / S molar ratios, weighed 2.0g and put it into the atmosphere tube furnace whose temperature had reached the specified temperature, calcined at different temperatures, and controlled different oxygen concentrations until there was no gas in the gas at the furnace outlet. SO 2 After degassing, stop the experiment.
[0088] The result is shown in Figure 3.
[0089] It can be seen from Figure 3(a) that under different oxygen concentrations, the corresponding optimal C / S is different. As the oxygen concentration increases, the minimum coal value required for reduction is greater. When the oxygen concentration is between 10 and 20%, before the coal value reaches the optimal C / S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com