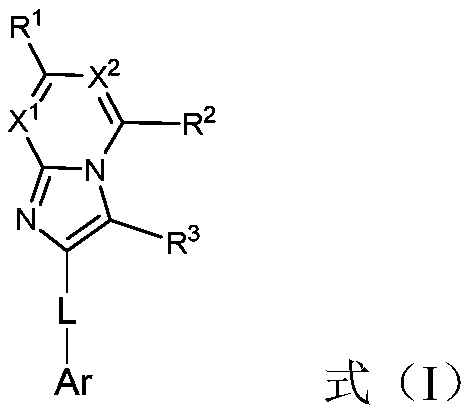
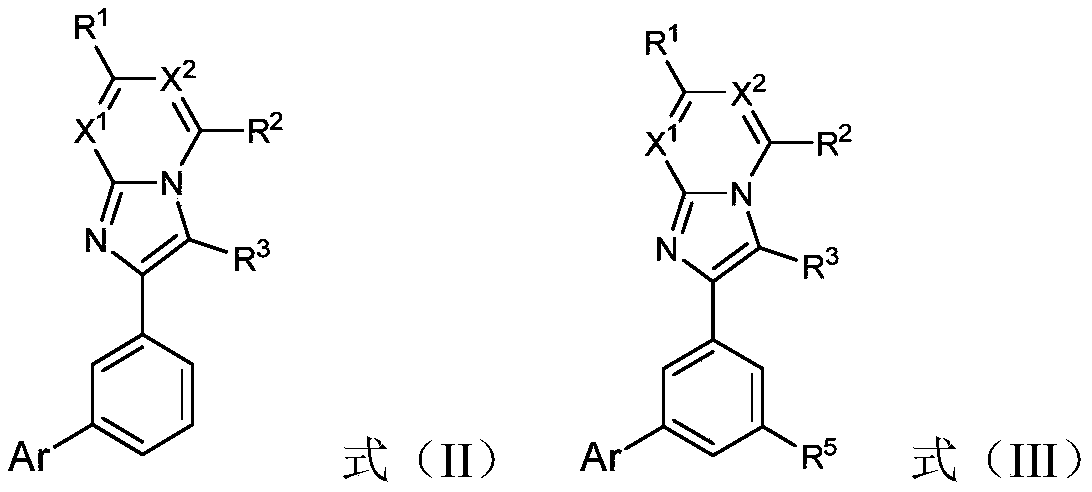
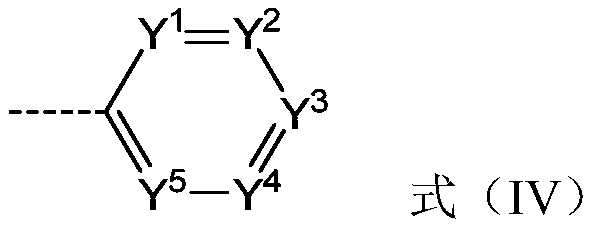
Compound, application thereof and organic electroluminescent device
A compound and selected technology, applied in the fields of electro-solid devices, electrical components, organic chemistry, etc., can solve the problem of electron injection ability and electron mobility need to be further optimized.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0126] Synthesis of compound C9:
[0127]
[0128] (1) Preparation of compound 1-1
[0129] At room temperature, under a nitrogen atmosphere, add 2-bromo-1-(3,5 -dichlorophenyl)ethanone (10.77g, 40.49mmol). After the addition, heat to reflux and stir for 5 hours. TLC detected that the reaction was complete, extracted with ethyl acetate, and washed the organic phase with saturated brine, dried the organic phase with anhydrous sodium sulfate, filtered, spin-dried under reduced pressure, and obtained compound 1-1 (13.44 g, yield 80%) by column chromatography .
[0130] (2) Preparation of compound 1-2
[0131] 1-1 (13.44g, 32.39mmol), 3-cyanophenylboronic acid (4.76g, 32.39mmol), potassium carbonate (13.41g, 97.17mmol), tetrakistriphenylphosphopalladium (0.81g, 0.65 mmol), 150 mL of solvent toluene, 30 mL of ethanol, and 30 mL of water were added, and the reaction was refluxed overnight at 80° C. under nitrogen replacement three times under protection. A solid precipitate...
Synthetic example 2
[0137] Synthesis of compound C28
[0138]
[0139] (1) Preparation of compound 2-1
[0140] Add 2-amino-4-chloro-6-phenyl-1,3,5-triazine (10.0g, 48.54mmol), 9,9-dimethylfluorene-3-boronic acid (11.55g, 48.54 mmol), potassium carbonate (20.09g, 145.63mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (0.71g, 0.97mmol), add solvent toluene 100mL, ethanol 20ml , 20 mL of water, and reflux at 80° C. overnight under nitrogen replacement three times and protection. A solid precipitated out during the reaction. It was detected by TLC that the reaction of the raw materials was complete, the reaction was stopped and cooled to room temperature, the precipitated solid was filtered, rinsed with water and ethanol respectively, and dried. The target compound 2-1 (10.60 g, yield 60%) was obtained.
[0141] (2) Preparation of compound 2-2
[0142] 2-Bromo-1-(3-bromophenyl)ethanone (8.04g, 29.12mmol) was added to 2-1 (10.60g, 29.12mmol) in DMF solution (20ml) under nitro...
Synthetic example 3
[0148] Synthesis of compound C46:
[0149]
[0150] (1) Preparation of compound 3-1
[0151] At room temperature, add 2-bromo-1-( 3,5-Dichlorophenyl)ethanone (11.13 g, 40.32 mmol). After the addition, heat to reflux and stir for 5 hours. TLC detected that the reaction was complete, extracted with ethyl acetate, and washed the organic phase with saturated brine, dried the organic phase with anhydrous sodium sulfate, filtered, spin-dried under reduced pressure, and obtained compound 3-1 (13.74 g, yield 80%) by column chromatography .
[0152] (2) Preparation of Compound C46
[0153] Add 3-1 (13.74g, 32.26mmol), 2-(3-boronate phenyl)-4,6-diphenyl-1,3,5-triazine (14.03g, 32.26mmol ), potassium carbonate (13.35g, 96.77mmol), tetrakistriphenylphosphopalladium (0.81g, 0.65mmol), add 150mL of solvent toluene, 30ml of ethanol, 30mL of water, replace with nitrogen three times and react at 80°C under protection and reflux overnight. A solid precipitated out during the reaction. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com