Novel falecalcitriol tablet and preparation method thereof
The technology of fluorocalcitriol tablets and fluorocalcitriol is applied in the directions of bone diseases, pharmaceutical formulations, medical preparations with inactive ingredients, etc., and can solve the problems such as difficult storage of fluorocalcitriol, drug deterioration, and influence on treatment effects. , to achieve the effect of maintaining stability, rapid decomposition and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
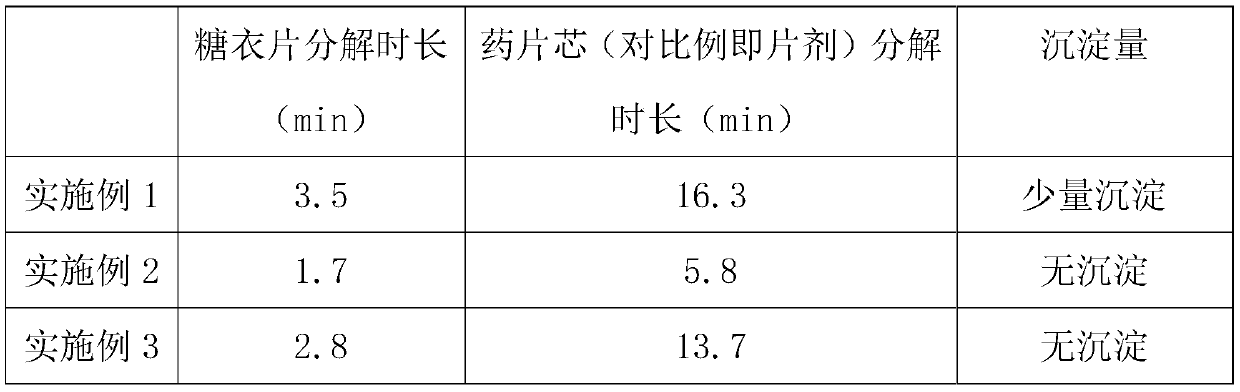
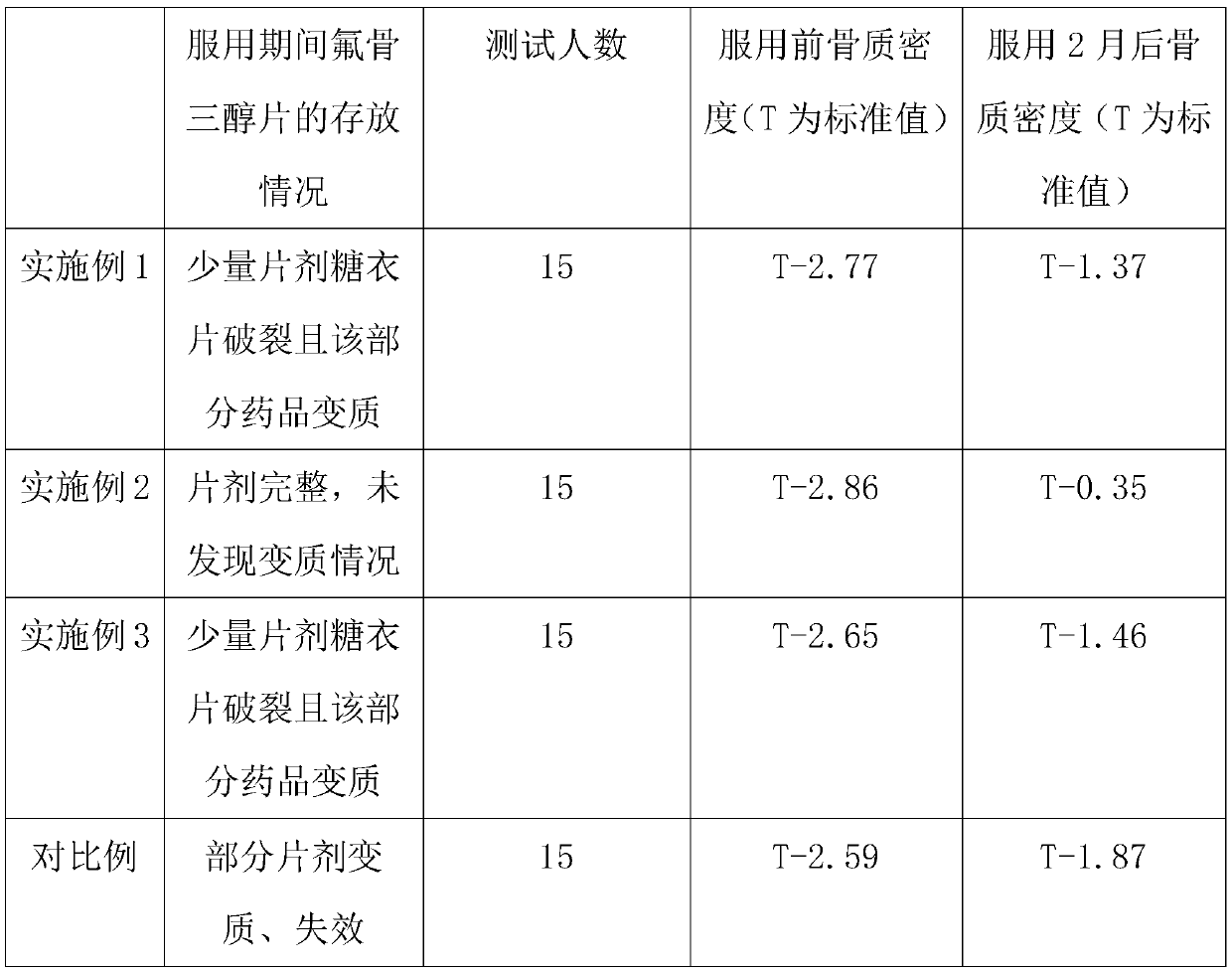
Embodiment 1
[0029] The invention provides a novel fluocautotriol tablet, comprising a tablet core and a sugar-coated tablet, wherein the tablet core includes the following raw materials in parts by weight: 0.01-0.06 parts of fluocautotriol, 12-18 parts of light calcium carbonate, 6-9 parts of calcium gluconate, 8-12 parts of manganese gluconate, 4-6 parts of anhydrous citric acid, 4072-3.5 parts of poloxamer, 0.8-1.2 parts of fat replenishing agent, the sugar-coated tablet is by weight The count includes: 4-6 parts of dextrin, 6-8 parts of medicinal gelatin, 0.5-1.5 parts of sodium carboxymethyl starch, 0.3-0.7 parts of polysorbate 80, 8-12 parts of talcum powder, and the balance is distilled water;
[0030] Specifically in this embodiment, the tablet core includes the following raw materials in parts by weight: 0.06 parts of fluocautotriol, 12 parts of light calcium carbonate, 6 parts of calcium gluconate, 8 parts of manganese gluconate, anhydrous citron 4 parts of acid, 2 parts of polox...
Embodiment 2
[0045] The invention provides a novel fluocautotriol tablet, comprising a tablet core and a sugar-coated tablet, wherein the tablet core includes the following raw materials in parts by weight: 0.01-0.06 parts of fluocautotriol, 12-18 parts of light calcium carbonate, 6-9 parts of calcium gluconate, 8-12 parts of manganese gluconate, 4-6 parts of anhydrous citric acid, 4072-3.5 parts of poloxamer, 0.8-1.2 parts of fat replenishing agent, the sugar-coated tablet is by weight The count includes: 4-6 parts of dextrin, 6-8 parts of medicinal gelatin, 0.5-1.5 parts of sodium carboxymethyl starch, 0.3-0.7 parts of polysorbate 80, 8-12 parts of talcum powder, and the balance is distilled water;
[0046] And specifically in this embodiment, wherein the tablet core includes the following raw materials in parts by weight: 0.03 parts of fluocautotriol, 15 parts of light calcium carbonate, 7.5 parts of calcium gluconate, 10 parts of manganese gluconate, anhydrous citron 5 parts of acid, 3...
Embodiment 3
[0061] The invention provides a novel fluocautotriol tablet, comprising a tablet core and a sugar-coated tablet, wherein the tablet core includes the following raw materials in parts by weight: 0.01-0.06 parts of fluocautotriol, 12-18 parts of light calcium carbonate, 6-9 parts of calcium gluconate, 8-12 parts of manganese gluconate, 4-6 parts of anhydrous citric acid, 4072-3.5 parts of poloxamer, 0.8-1.2 parts of fat replenishing agent, the sugar-coated tablet is by weight The count includes: 4-6 parts of dextrin, 6-8 parts of medicinal gelatin, 0.5-1.5 parts of sodium carboxymethyl starch, 0.3-0.7 parts of polysorbate 80, 8-12 parts of talcum powder, and the balance is distilled water;
[0062] Specifically in this embodiment, the tablet core includes the following raw materials in parts by weight: 0.01 part of fluocautotriol, 18 parts of light calcium carbonate, 9 parts of calcium gluconate, 12 parts of manganese gluconate, anhydrous citron 6 parts of acid, 3.5 parts of pol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com