Drug carrier, brain-targeted nanomedicine based on CRISPR gene editing technology and its preparation method and application
A nano-drug and gene editing technology, applied in drug combination, drug formulation, drug delivery, etc., can solve the problems of high recurrence rate, high mortality rate, low cure rate, etc., and achieve enhanced stability, good biocompatibility, The effect of high cutting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
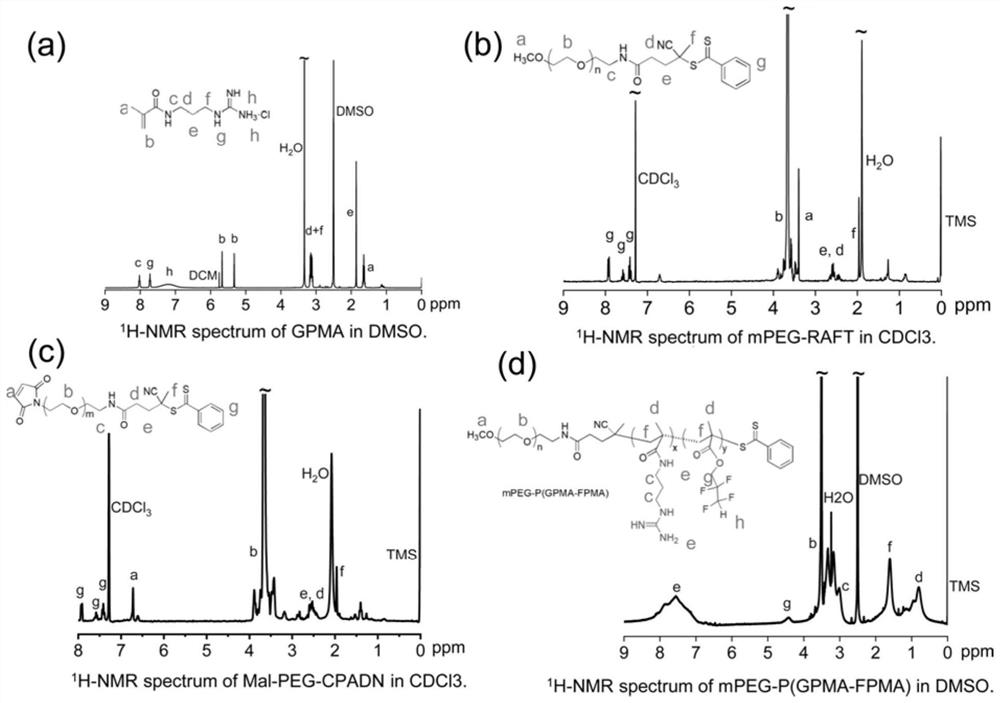
[0048] The present invention provides a drug carrier, a brain-targeted nanomedicine based on CRISPR gene editing technology and its preparation method and application. Specifically, the present invention provides the following technical solutions.
[0049] The first aspect of the present invention provides a drug carrier, including polymer mPEG-P (GPMA, FPMA) and polymer Ang-PEG-PGPMA;
[0050] The structural formula of the mPEG-P (GPMA, FPMA) is:
[0051]
[0052] The structural formula of the polymer Ang-PEG-PGPMA is:
[0053]
[0054] Wherein, n is 35-45, x1 is 15-20, y is 2-4, m is 75-85, and x2=x1.
[0055] The Angiopep polypeptide in the drug carrier of the present invention helps to pass through the BBB and target to tumor cells, PEG can effectively prolong the blood circulation cycle and has good biocompatibility, and the guanidino Gu + It can not only combine with riboprotein complexes through electrostatic interaction but also form salt bridges and hydrogen b...
Embodiment 1
[0076] Preparation method of nano medicine
[0077] In this example, the nanomedicine is the nanoparticle Ang-NP@Cas9 / sgRNA, and the preparation method of the nanoparticle includes: transcribing sgRNA in vitro, synthesizing a polymer, and preparing nanoparticles. The target sequence of the transcribed sgRNA is TACCTACGGCAAATTGTGCT (SEQ ID NO: 1). Specific steps are as follows:
[0078] 1. In vitro transcription of sgRNA
[0079] In vitro transcription mimics the environment of in vivo transcription, and there is no RNase during the entire operation process. The purpose is to prevent the degradation of sgRNA after transcription. The samples should be divided and stored in a -80°C refrigerator. Specific steps are as follows:
[0080] 1.1 Annealed oligosaccharide oligo (total system 20μL)
[0081]
[0082] 95°C, after 5 minutes, leave it at room temperature overnight (or cool it down to room temperature naturally in boiling water).
[0083] 1.2 Add 1 μL of Taq enzyme (10X...
Embodiment 2
[0127] Nanomedicine Characterization Results
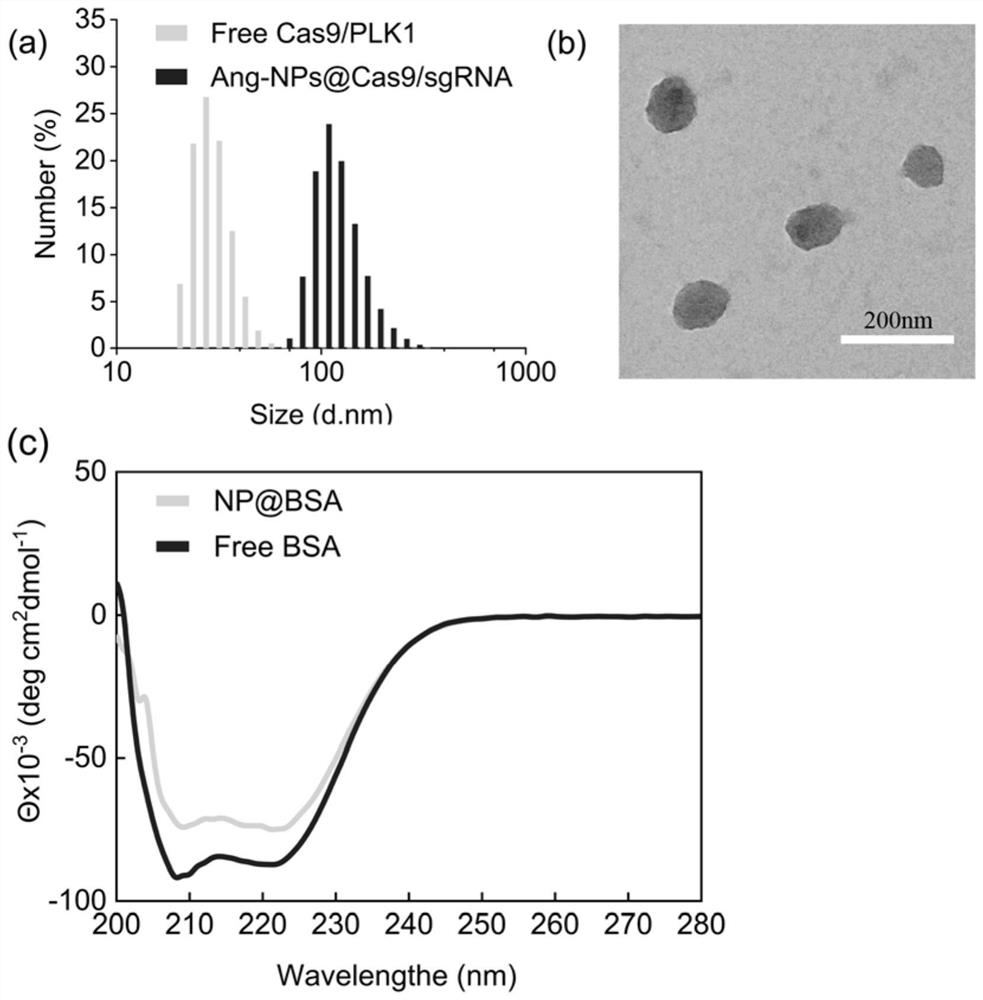
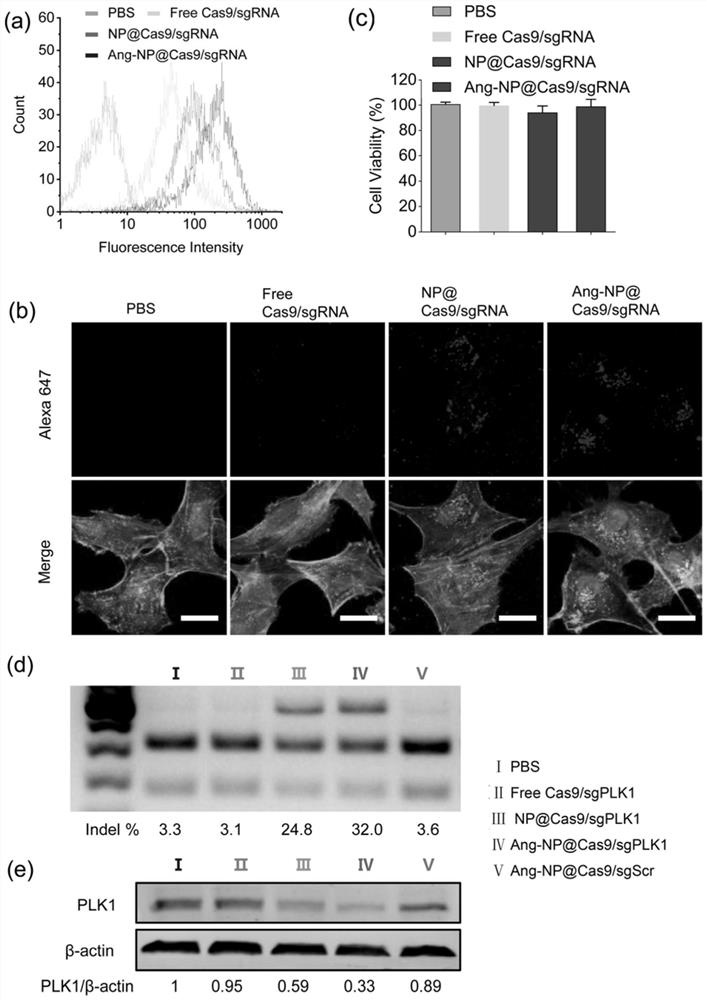
[0128] The particle size diagram of Ang-NP@Cas9 / sgPLK1 and Free Cas9 / sgPLK1 (i.e. Cas9 / sgPLK1 complex) is shown in figure 2 As shown in (a), the particle size and potential of the nanomedicine are shown in Table 1. figure 2 Middle (b) is the TEM image of Ang-NP@Cas9 / sgPLK1. The particle size of the Cas9 / sgPLK1 complex is about 30nm, with a negative charge. After being wrapped by a polymer, the particle size is 149nm, the stability is significantly enhanced, the uniformity is very good, and the surface has a weak positive charge. Treating tumors offers possibilities.
[0129] Table 1 The particle size and potential of nanomedicine
[0130]
[0131]
[0132] figure 2 Middle (c) is the detection result of circular dichroism spectrum. In this embodiment, BSA protein is taken as an example, using mPEG 2K -P(GPMA 4K ,FPMA 0.6K ) to prepare nanomedicine (NPs-BSA) by loading BSA protein, and compare the peak changes of Fre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com