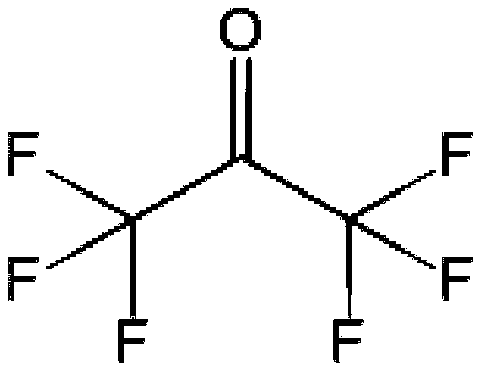
Novel process for synthesizing hexafluoroacetone
A technology of hexafluoroacetone and a new process, which is applied in the field of flame retardants, can solve the problems of high production cost, high price of hexafluoropropylene oxide, and limited use, and achieve high reaction yield, easy industrial production, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 210g (1mol) trifluoroacetic anhydride, 1200mL tetrahydrofuran and 2mol sodium fluoride were added to the reactor, reacted at 20°C for 3 hours, then 1.1mol potassium carbonate and 2.1mol sodium trifluoroacetate were added to the reactor, and the temperature was raised to 120 °C. ℃, stirring and reacting for 3 hours, cooling down, opening the reaction kettle, and distilling to obtain 435.3 g of the product hexafluoroacetone trihydrate. After testing, the HPLC purity was 96.3%, and the product yield was 93.1%.
Embodiment 2
[0022] 210g (1mol) trifluoroacetic anhydride, 1800mL dioxane and 2mol potassium fluoride were added in the reactor, reacted at 50°C for 1 hour, then added 1.1mol calcium carbonate and 2.1mol potassium trifluoroacetate in the reactor , heated to 70°C, stirred for 10 hours, cooled, opened the reactor, and distilled to obtain 438.3g of hexafluoroacetone trihydrate. After testing, the HPLC purity was 96.1%, and the product yield was 95.7%.
Embodiment 3
[0024] 210g (1mol) trifluoroacetic anhydride, 2000mL diethylene glycol dimethyl ether and 2.3mol cesium fluoride were added to the reaction kettle, reacted at 50°C for 2 hours, then 1.2mol cesium carbonate and 2.3mol trifluoride were added to the reaction kettle. Lithium fluoroacetate was heated to 80°C, stirred and reacted for 7 hours, cooled, opened the reaction kettle, and distilled to obtain 429.2 g of the product hexafluoroacetone trihydrate. After testing, the HPLC purity was 98.3%, and the product yield was 95.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com