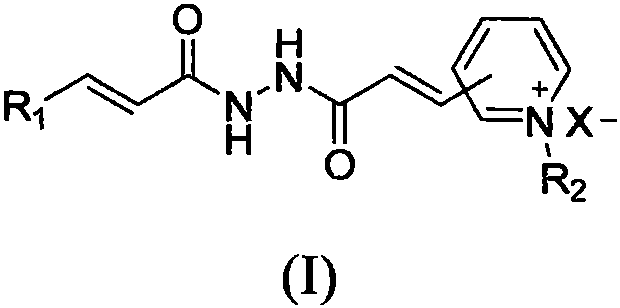
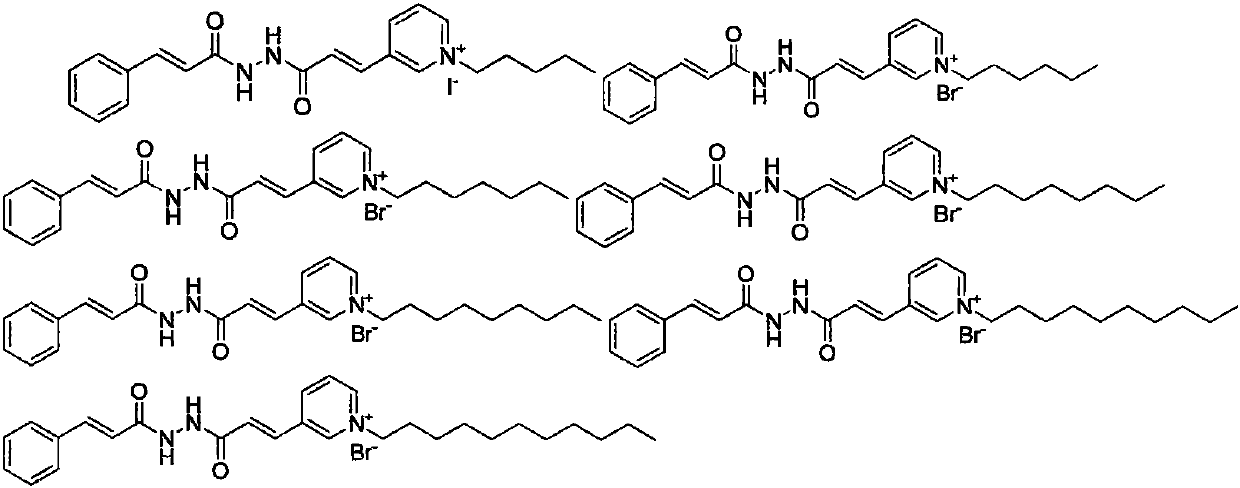
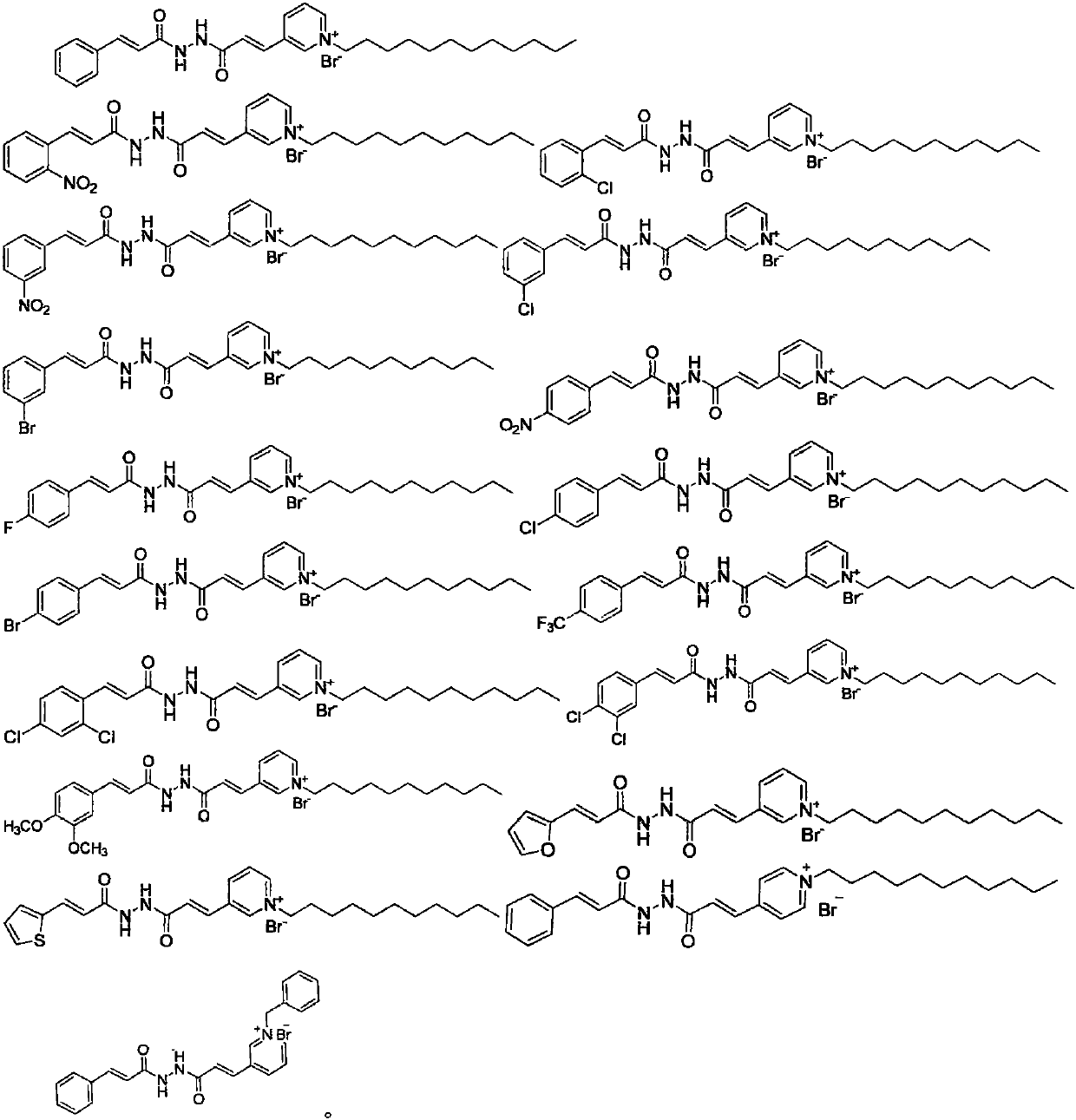
Pyridinium salt-containing N-(cinnamoyl)-N'-(substituted) propyl hydrazide compound, preparation method and application thereof
A technology of cinnamoyl and propyl hydrazide, which is applied in the field of propyl hydrazide compounds and their preparation, and can solve problems affecting citrus yield and citrus rot
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the synthesis of 3-(3-pyridine) acrylic acid hydrazide
[0054] Add 3-(3-pyridine)acrylic acid (6.70mmol), EDCI (7.38mmol), HoBt (7.38mmol) into a 500mL round bottom flask, add 5mL of acetonitrile, add about 0.5mL of triethylamine under stirring to dissolve, add 300mL of acetonitrile, then slowly dropwise added 1mL of 60% hydrazine hydrate, and react overnight at room temperature. After the reaction is complete, filter with suction to remove the solids. After the filtrate is decompressed and precipitated, add 5 mL of 10% NaOH aqueous solution and extract with EA to remove unreacted 3-(3-pyridine) acrylic acid. Use anhydrous sodium sulfate for the organic phase After drying, the sample was mixed with silica gel, and the gradient elution of DCM:MeOH=10:1~5:1 was used to purify a yellow solid with a yield of 40%.
[0055] Other hydrazide intermediate compounds are synthesized according to the steps of Example 1 using corresponding raw materials or substituen...
Embodiment 2
[0056] Example 2: Synthesis of (E)-N'-cinnamoyl-3-pyridin-3-yl)acrylhydrazide
[0057] Add cinnamic acid (1.88mmol), EDCI (2.26mmol), HoBt (2.26mmol) into a 25mL round bottom flask, add 15mL of acetonitrile, add about 0.1mL of triethylamine to dissolve it under stirring, then add 3-(3- pyridine) acrylic acid hydrazide (1.88mmol), react at room temperature overnight. After the reaction was completed, it was suction filtered to obtain a solid, which was dispersed by heating with dichloromethane as a solvent. After cooling, it was suction filtered to obtain a yellow solid with a yield of 55%.
[0058] Other (E)-N'-(substituted) cinnamoyl-(3-pyridin-3-yl)propenyl hydrazide intermediate compounds were synthesized by referring to the steps of Example 2 using corresponding raw materials or substituents.
Embodiment 3
[0059] Embodiment 3: Preparation of the pyridinium salt compound of (E) N'-cinnamoyl-3-(pyridin-3-yl)acrylohydrazide structure
[0060] Add (E)-N'-cinnamoyl-(3-pyridin-3-yl)acrylhydrazide (1.02mmol) into a 15mL round-bottomed flask, add 5mL DMF, heat to 100°C to dissolve, and wait to dissolve After adding the corresponding halogenated hydrocarbon (1.23mmol), react at 110-120°C for about 4h. After the reaction is complete, cool to precipitate a solid, add a small amount of ethyl acetate while stirring, then filter with suction, wash the filter cake with ethyl acetate, dry, and recrystallize from methanol to obtain the target product.
[0061] Other target product compounds are synthesized with reference to the steps of Example 3 using corresponding raw materials or substituents.
[0062] The synthetic part of N-(cinnamoyl)-N'-(substituted) propylhydrazide compounds containing pyridinium salts and the H-NMR and C-NMR data are shown in Table 1, and the physicochemical properties...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com