Beta-sitosterol derivative as well as preparation method and application thereof
A technology of β-sitosterol and its derivatives, which is applied in the field of β-sitosterol derivatives and its preparation, can solve the problem of the solubility of β-sitosterol and the specific combination of β-sitosterol derivatives and avidin to construct nanoparticles, etc. problems, to achieve good clinical application prospects, convenient blood vessel administration, and convenient preparation of pharmaceutical dosage forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
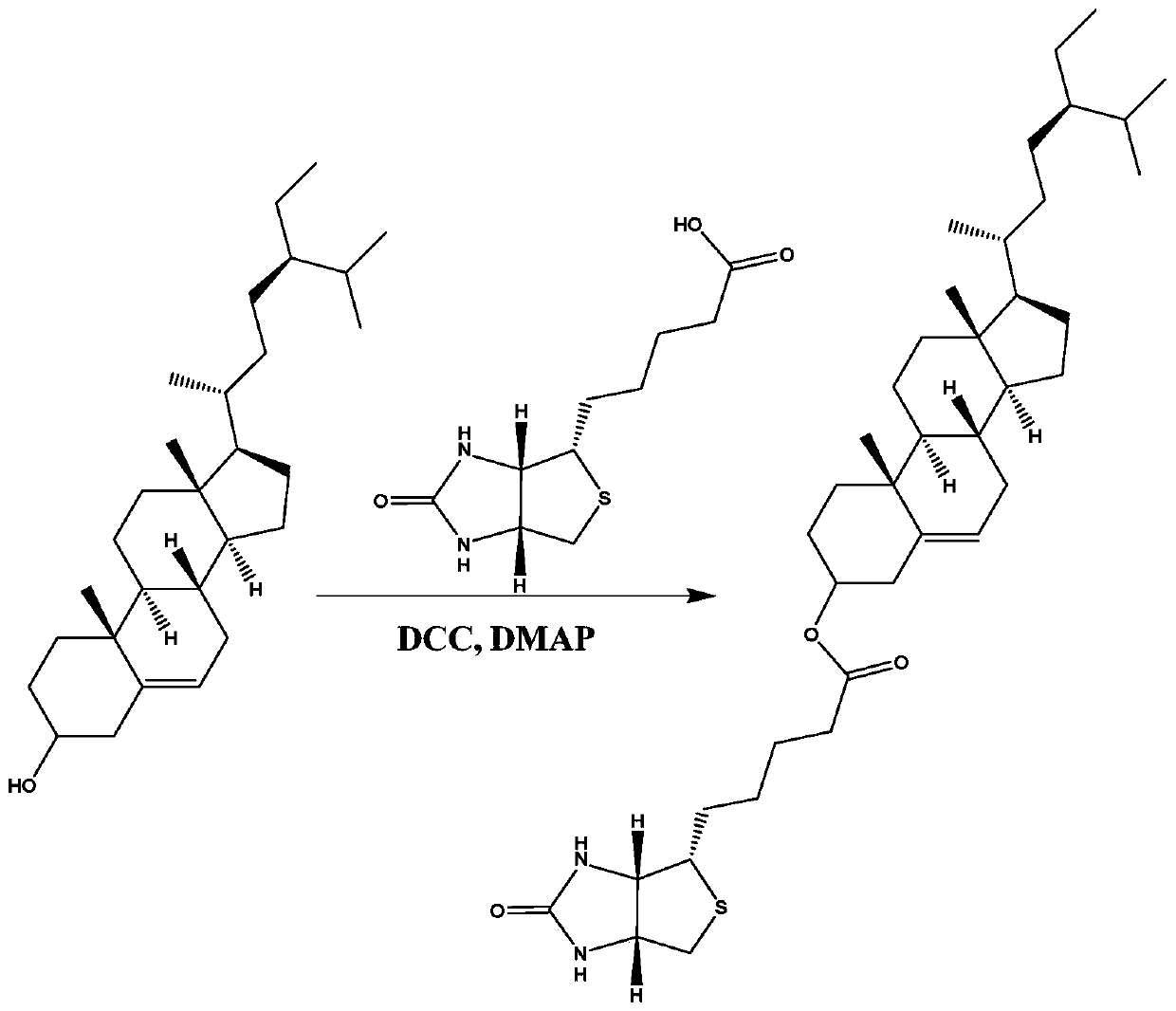
[0036] Embodiment 1 Preparation of β-sitosterol derivatives (B-BS) of the present invention
[0037] Dissolve β-sitosterol (0.15mmol) in 60ml of DMSO, under the action of dehydrating agent DCC (3.0mmol) and catalyst DMAP (3.0mmol), stir at 0°C for 6h; then add β-sitosterol at a molar ratio of 1:3 For biotin (B), raise the temperature from the ice bath to room temperature 25°C, and stir overnight in the dark. Concentrate the filtrate, recrystallize with glacial ether or isopropanol, purify by chromatography or preparative liquid phase, and freeze-dry to obtain the β-sitosterol derivative B-BS (yield is about 48%). The ion peak identified by mass spectrometry [M+H ] + For: 642.1.
[0038] 1 HNMR (400MHz, DMSO): The characteristic peaks of biotin: 10.76-10.82 (NH, 2H, broad peak), 4.47-4.65 (2C-H, 2H), 2.80-3.10 (CH 2 , 2H), 3.24(CH, 1H), 2.32(CH 2 , 2H), 1.23-1.68 (3CH 2 , 6H); The characteristic peak of β-sitosterol: 0.85-0.88 (4CH 3 , 12H), 0.99-1.03 (CH 3 , 3H), 1.08-...
Embodiment 2
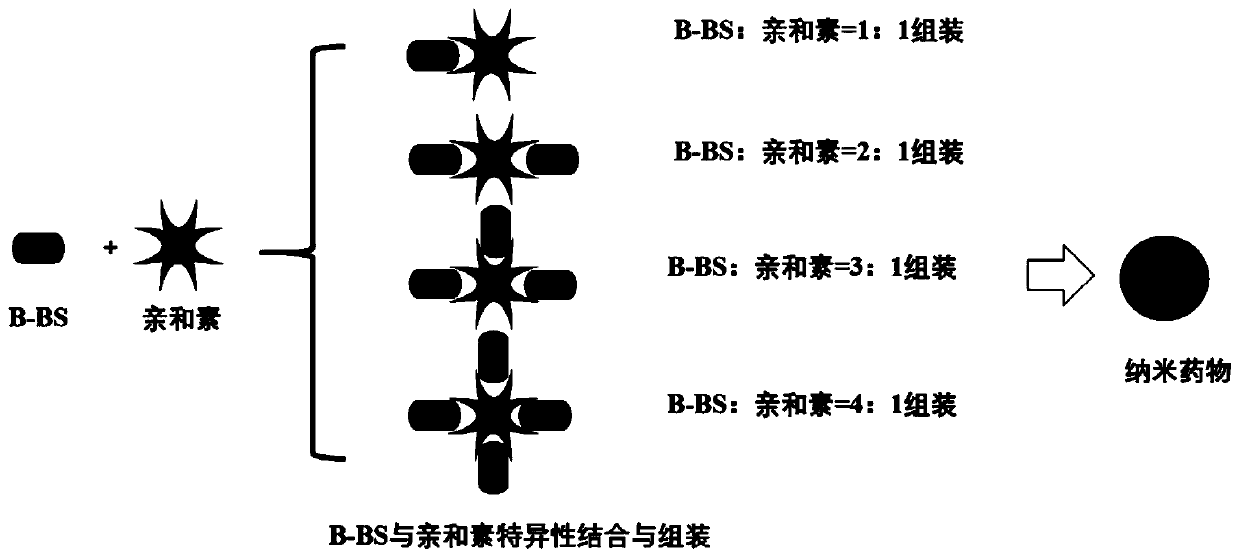
[0042] Preparation of embodiment 2β-sitosterol derivative avidin nanoparticles
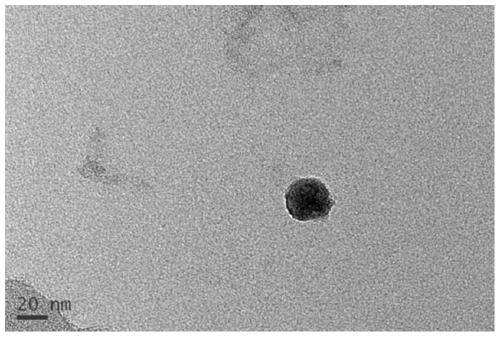
[0043] Get the β-sitosterol derivative (B-BS) that is equivalent to β-sitosterol 5g and prepare through embodiment 1, be dissolved in a small amount of DMSO or ethanol earlier, dilute with water for injection then, add sodium chloride 1.0g, then Add equimolar avidin (the assembly of B-BS and avidin can be assembled according to various molar ratios of 1-4:1, such as figure 2 shown), stir evenly at room temperature to obtain composite β-sitosterol derivative avidin nanoparticles, characterized by transmission electron microscopy, its nanometer size is about 25nm (such as image 3 shown).
Embodiment 3
[0044] The preparation of embodiment 3β sitosterol derivatives injection
[0045] Take the β-sitosterol derivative (B-BS) prepared by Example 1 in an amount equal to 30 g of β-sitosterol, dissolve it in water for injection, add 5.0 g of sodium chloride and stir evenly, dilute hydrochloric acid to adjust the pH=5.0, and then add 0.5% activated carbon for injection, keep the temperature at 60°C for 30 minutes, after decarbonization, add water for injection to the filtrate to 1000ml, filter through a 0.22μm sterile filter membrane, pack 2ml / branch into glass curved neck ampoules, melt seal, and circulate at 100°C Steam damp heat sterilization for 40 minutes, and then label and store.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com