Treatment method for de-proliferation ability of feeder cells for NK cell culture
A feeder cell and NK cell technology, which is applied in the field of feeder cell deproliferation treatment, can solve the problems of affecting NK cell expansion effect, incomplete cell shape, and high test cost, so as to achieve convenient promotion and use, low cost, and high lethality active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Mitomycin C treatment method for feeder cells
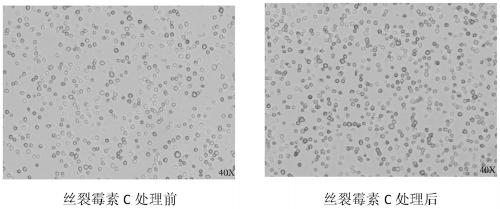
[0022] First add appropriate amount of DMSO to dissolve mitomycin C so that the concentration is 33 mg / ml, that is, prepare mitomycin C stock solution, aliquot and store at -20°C for later use. 1200rpm, 5min centrifugation to collect 41BBL-mbIL-21-K562 feeder cells, and use RPMI1640 medium to dilute the feeder cells to a concentration of 1x10 7 cells / ml of cell suspension. Add mitomycin C to the cell suspension to a final concentration of 33 μg / ml at 37 °C in 5% CO 2 After being treated in the dark for 90 minutes, the cells were collected at 1200 rpm for 5 minutes, and washed 4 times with DPBS to remove residual mitomycin C as much as possible to avoid toxicity to the cultured cells. The treated feeder cells can be frozen in cell freezing medium for future use, or directly used for NK cell expansion or other experiments.
[0023] In order to verify the proliferative ability of the feeder cells treated by the m...
Embodiment 2
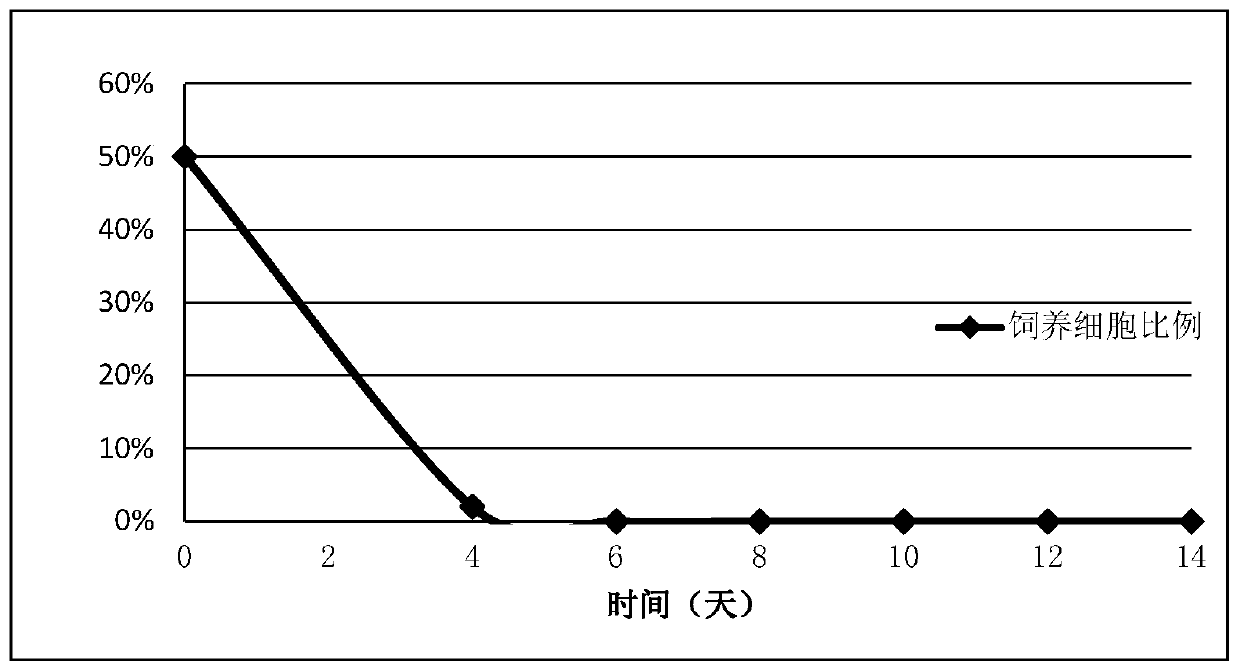
[0028] Embodiment 2 NK cells are cultured after 14 days feeder cell retention situation
[0029] The feeder cells used are K562 cells, and the ratio of feeder cells can be detected by flow cytometry with Anti-41BBL PE antibody (R&D product number FAB2295P).
[0030] 1. Isolation of PBMCs: Collect 50ml of peripheral blood from tumor patients, anticoagulate with heparin, and centrifuge whole blood at 700g for 20min. The upper layer is the plasma layer, the middle layer is the buffy coat layer, and the lower layer is red blood cells. The plasma layer was collected, inactivated at 56°C for 30min, then allowed to stand at 4°C for 15min, 1800rpm / 800g, 4°C for 25min, and the supernatant was used as autologous plasma for later use. Collect the middle buffy coat, add it to 20ml PBS, mix well, then slowly add the centrifuge tube that has been added with lymphatic separation solution at a ratio of 4:3, 1800rpm / 800g, room temperature, 20min, no-break, wash PBMC; Suck out the white PBMC ...
Embodiment 3
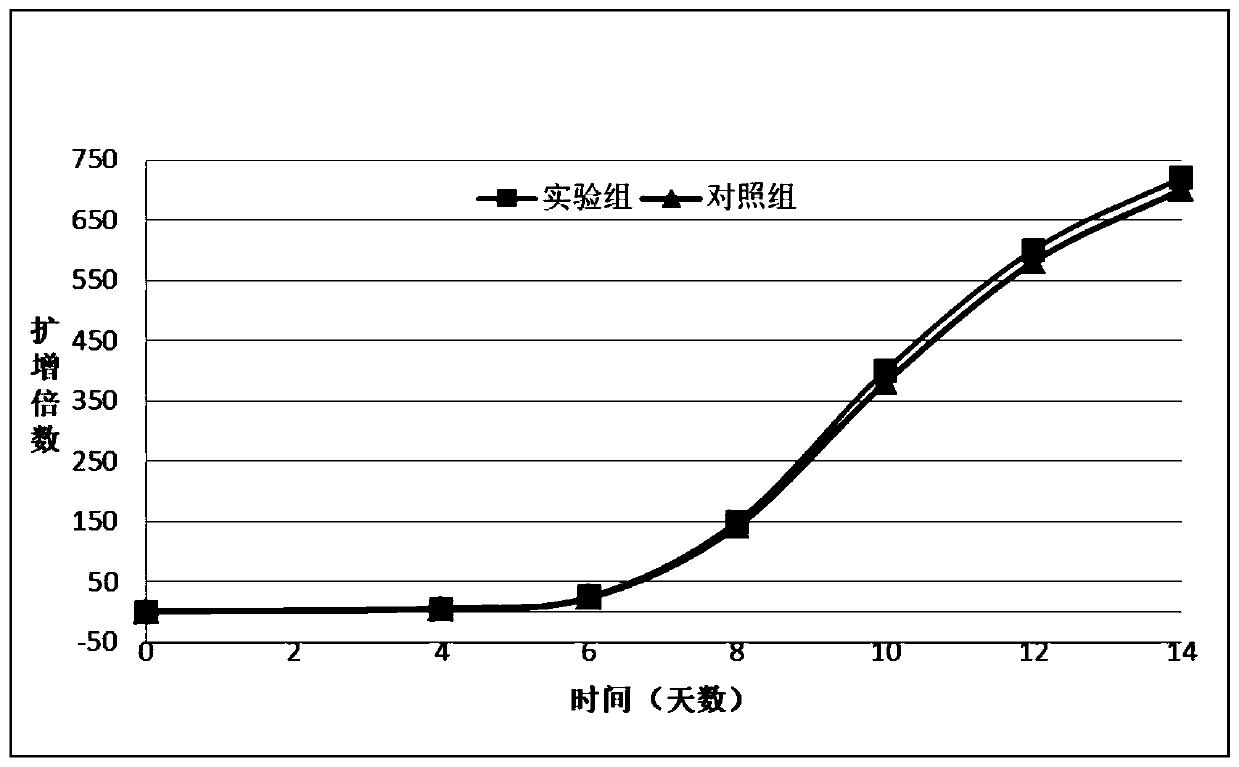
[0034] Example 3 Use of Treated Cells to Cultivate NK Cells
[0035] 1. Isolation of PBMCs
[0036] Collect 50ml of peripheral blood from tumor patients, anticoagulate with heparin, and centrifuge whole blood at 700g for 20min. The upper layer is the plasma layer, the middle layer is the buffy coat layer, and the lower layer is red blood cells. The plasma layer was collected, inactivated at 56°C for 30min, then allowed to stand at 4°C for 15min, 1800rpm / 800g, 4°C for 25min, and the supernatant was used as autologous plasma for later use. Collect the middle buffy coat layer, add it to 20ml PBS, mix well, then slowly add the centrifuge tube that has been added with lymphatic separation solution at a ratio of 4:3, 1800rpm / 800g, room temperature, 20min, no-break, wash PBMC; Suck out the white PBMC layer in the middle after centrifugation with a Sider pipette, add about 30ml of PBS to a new 50ml centrifuge tube to wash, 1500rpm, 8min, wash with culture medium again, and count.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com