Synthetic method for increasing yield of 5-chloro-1-indanone
A synthesis method and technology of indanone are applied in the synthesis field of improving the yield of 5-chloro-1-indanone, can solve the problems of many side reactions, poor selectivity, low yield and the like, and achieve improved selectivity, improved viscosity, The effect of reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
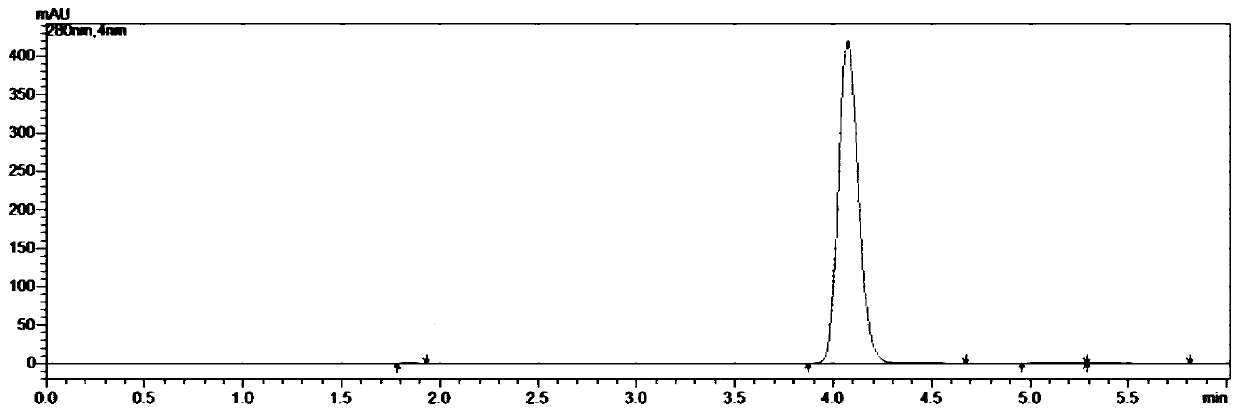
Embodiment 1
[0049] A kind of synthetic method of 5-chloro-1-indanone, the concrete steps are as follows:
[0050] (1) take by weighing 211.0g 3,4'-dichloropropiophenone, 400.5g aluminum trichloride and place in reaction flask, connect tail gas absorption device, open stirring, be slowly warming up to 100 ℃, and system presents molten state;
[0051] (2) When the system is in a molten state, weigh 21.1g of tetrabutylammonium bromide, add it to the reaction flask, continue to heat up, and heat up to 160°C for 1 hour; after reaching the target temperature, keep the temperature for 2 hours, and monitor the reaction by HPLC. When there is no 3,4'-dichloropropiophenone remaining, the reaction is stopped;
[0052] (3) Cool the reaction solution to 80°C, transfer it into 0-5°C water, and stir for 1 h;
[0053] (4) suction filtration, the filter cake is rinsed with water to filtrate pH=6~7, and suction filtration is carried out until no water droplets fall to obtain 337.2g of crude product;
[0...
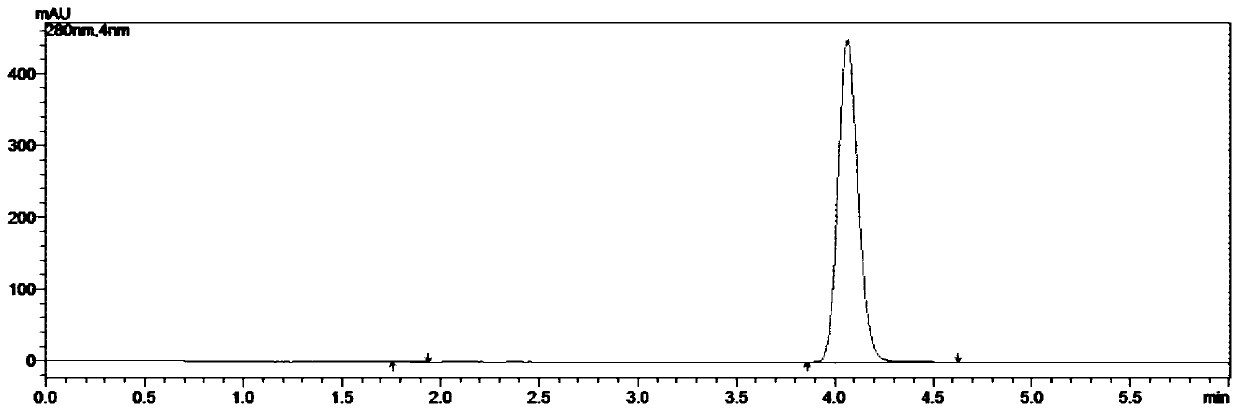
Embodiment 2
[0063] A kind of synthetic method of 5-chloro-1-indanone, the concrete steps are as follows:
[0064] (1) take by weighing 211.0g 3,4'-dichloropropiophenone, 400.5g aluminum trichloride and place in reaction flask, connect tail gas absorption device, open stirring, be slowly warming up to 100 ℃, and system presents molten state;
[0065] (2) When the system is in a molten state, weigh 1.06g of tetrabutylammonium bromide, add it to the reaction flask, continue to heat up, and heat up to 160°C for 1 hour; after reaching the target temperature, the reaction is incubated for 4.5 hours, and the reaction is monitored by HPLC. At this time, there is no 3,4'-dichloropropiophenone remaining, and the reaction is stopped;
[0066] (3) Cool the reaction solution to 80°C, transfer it into 0-5°C water, and stir for 1 h;
[0067] (4) suction filtration, the filter cake is rinsed with water to filtrate pH=6~7, and suction filtration is carried out until no water droplets fall to obtain 311.8...
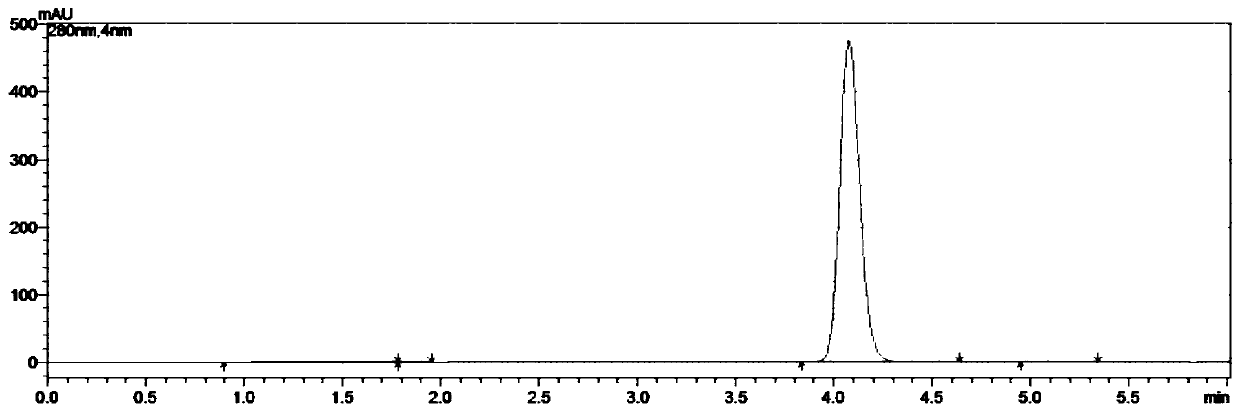
Embodiment 3
[0073] A kind of synthetic method of 5-chloro-1-indanone, the concrete steps are as follows:
[0074] (1) take by weighing 211.0g 3,4'-dichloropropiophenone, 400.5g aluminum trichloride and place in reaction flask, connect tail gas absorption device, open stirring, be slowly warming up to 100 ℃, and system presents molten state;
[0075] (2) When the system is in a molten state, weigh 21.1 g of tetrabutylammonium chloride, add it to the reaction flask, continue to heat up, and heat up to 160 ° C for 1 hour; after reaching the target temperature, the reaction is kept for 4 hours, and the reaction is monitored by HPLC. When there is no 3,4'-dichloropropiophenone remaining, the reaction is stopped;
[0076] (3) Cool the reaction solution to 80°C, transfer it into 0-5°C water, and stir for 1 h;
[0077] (4) suction filtration, the filter cake is rinsed with water to the pH of the filtrate=6~7, and the suction filtration is carried out until no water droplets fall to obtain 317.6g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com