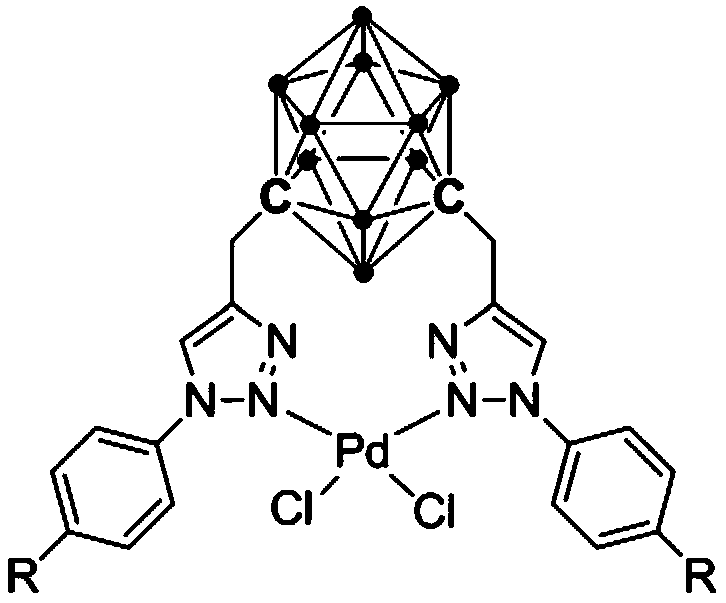
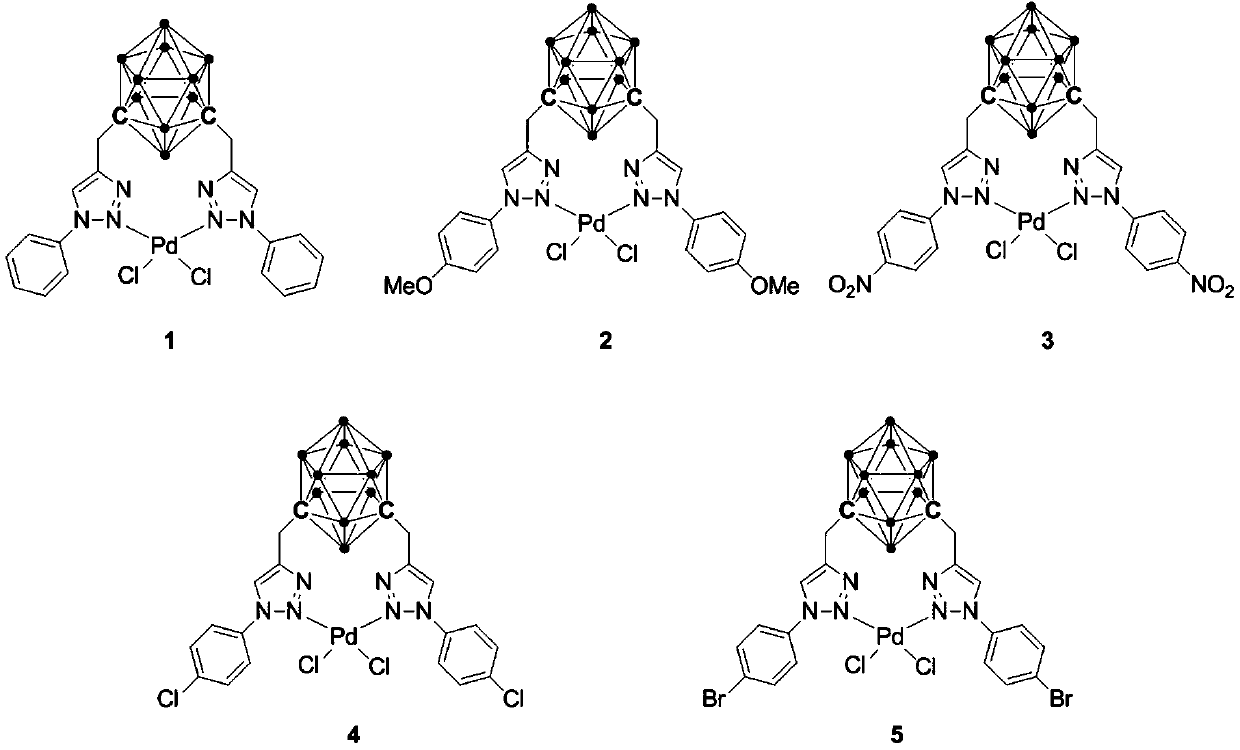
Palladium complex containing meta-position carborane triazole ligand and preparation method and application of palladium complex
A technology for meta-carborane and dipropargyl meta-carborane, which is applied in the field of complex synthesis, can solve the problems of harsh reaction conditions, long reaction time and the like, and achieves mild reaction conditions, simple preparation method, and wide industrial application. The effect of the application foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Synthesis of palladium complex 1 and its application in the coupling reaction of catalyzed mercaptans and halogenated hydrocarbons
[0035] (1) At 0°C, add n-BuLi (22.0mmol) n-hexane solution dropwise to m-C containing m-carborane 2 B 10 h 12 (10.0mmol) in ether solution, continue to stir for 30 minutes after the dropwise addition, slowly rise to room temperature and continue to react for 30 minutes, then add 3-bromopropyne (21.0mmol), continue to react at room temperature for 3 hours, after the reaction ends The solvent was drained, and the product was recrystallized from n-hexane to obtain 1,3-dipropargyl m-carborane C 8 B 10 h 16 (productive rate 85%), reaction equation is:
[0036]
[0037] 1 H NMR (400MHz, CDCl 3 ,25°C): δ=3.55(s,2H),2.63(s,4H).Theoretical value of elemental analysis C 8 B 10 h 16 : C 43.61, H 7.32; Found: C 43.55, H 7.30.
[0038] (2) CuI (0.05mmol), 1,3-dipropargyl m-carborane (1.0mmol) and benzene azide (1.2mmol) were...
Embodiment 2
[0044] Embodiment 2: the synthesis of palladium complex 2
[0045] (1) At 0°C, add n-BuLi (22.0mmol) n-hexane solution dropwise to m-C containing m-carborane 2 B 10 h 12 (10.0mmol) in ether solution, continue to stir for 30 minutes after the dropwise addition, slowly rise to room temperature and continue to react for 30 minutes, then add 3-bromopropyne (21.0mmol), continue to react at room temperature for 3 hours, after the reaction ends The solvent was drained, and the product was recrystallized from n-hexane to obtain 1,3-dipropargyl m-carborane C 8 B 10 h 16 (productive rate 85%), reaction equation is:
[0046]
[0047] 1 H NMR (400MHz, CDCl 3 ,25°C): δ=3.55(s,2H),2.63(s,4H).Theoretical value of elemental analysis C 8 B 10 h 16 : C 43.61, H 7.32; Found: C 43.55, H 7.30.
[0048] (2) Dissolve CuI (0.05mmol), 1,3-dipropargyl m-carborane (1.0mmol) and 4-methoxyazidebenzene (1.5mmol) in THF at room temperature, and at this temperature React for 5 hours, then PdCl...
Embodiment 3
[0051] Embodiment 3: the synthesis of palladium complex 3
[0052] (1) At 0°C, add n-BuLi (22.0mmol) n-hexane solution dropwise to m-C containing m-carborane 2 B 10 h 12 (10.0mmol) in ether solution, continue to stir for 30 minutes after the dropwise addition, slowly rise to room temperature and continue to react for 30 minutes, then add 3-bromopropyne (21.0mmol), continue to react at room temperature for 3 hours, after the reaction ends The solvent was drained, and the product was recrystallized from n-hexane to obtain 1,3-dipropargyl m-carborane C 8 B 10 h 16 (productive rate 85%), reaction equation is:
[0053]
[0054] 1 H NMR (400MHz, CDCl 3 ,25°C): δ=3.55(s,2H),2.63(s,4H).Theoretical value of elemental analysis C 8 B 10 h 16 : C 43.61, H 7.32; Found: C 43.55, H 7.30.
[0055] (2) Dissolve CuI (0.05mmol), 1,3-dipropargyl m-carborane (1.0mmol) and 4-nitroazidobenzene (1.3mmol) in THF at room temperature, and react at this temperature 5 hours, then PdCl 2 (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com