Method for determining photodegradation impurities in levofloxacin raw material and levofloxacin preparation
A levofloxacin and photodegradation technology, which is applied in the direction of measuring devices, material separation, and analysis of materials, can solve the problems that cannot meet the limit detection sensitivity requirements and the low response value of diformyl impurities, and is conducive to safe promotion and application. The effect of good accuracy and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
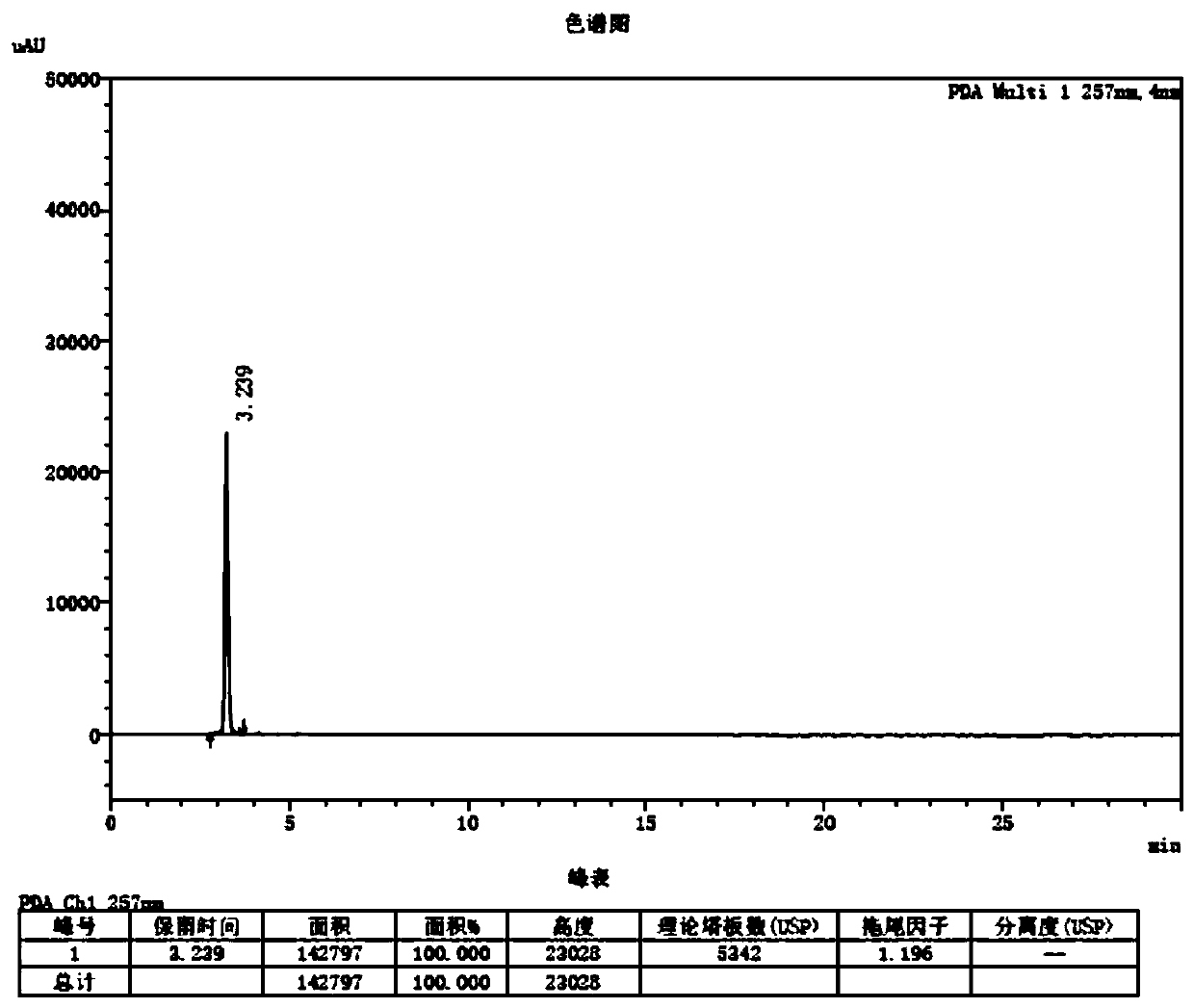
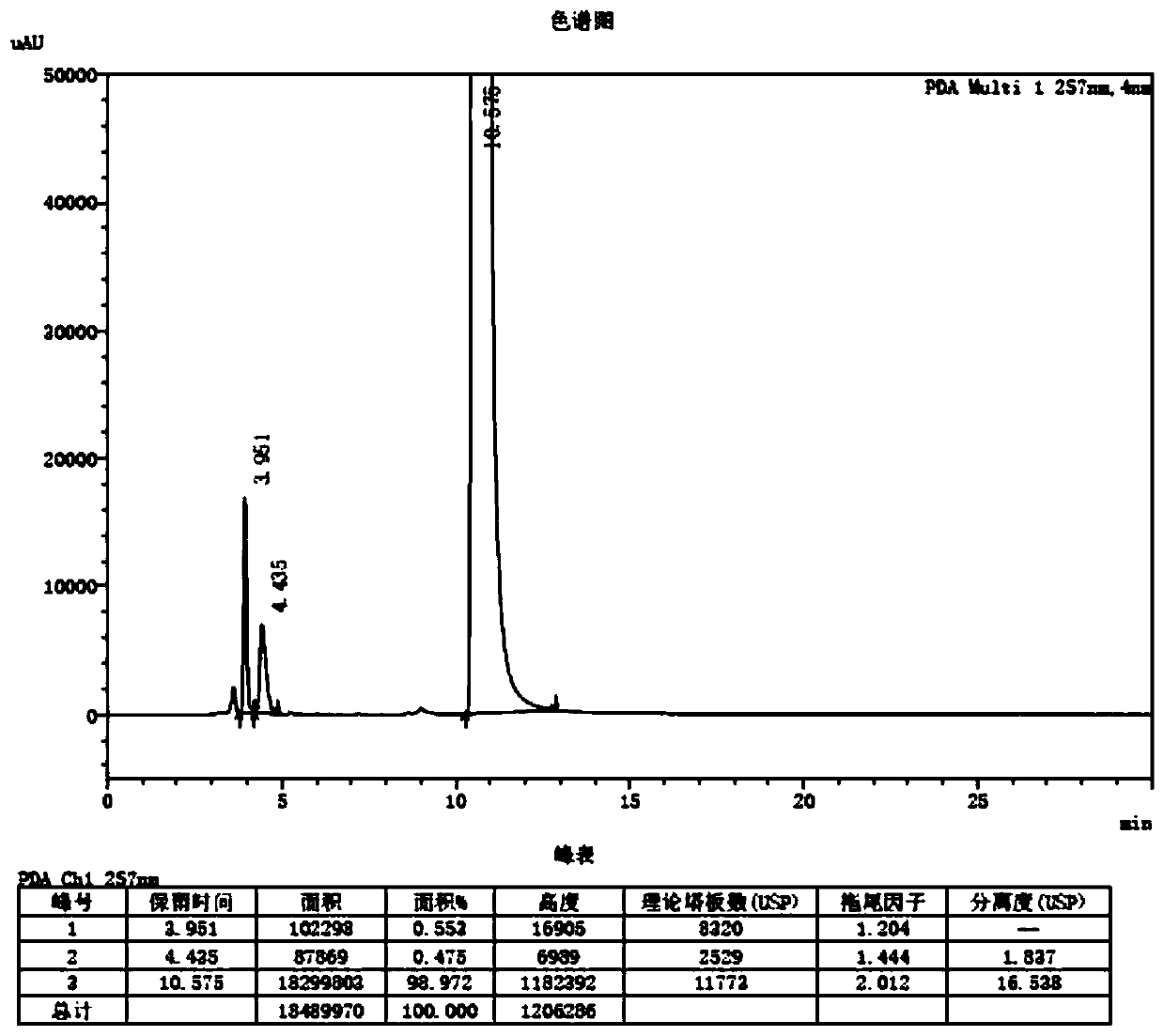
[0041] The method for measuring photodegradation impurities in levofloxacin raw materials and preparations is measured by high performance liquid chromatography, and the chromatographic conditions are as follows:
[0042] Chromatographic column: use aminosilane-bonded silica gel as filler, 4.6×250mm, 5μm;
[0043] Mobile phase: acetonitrile-0.05mol / L potassium dihydrogen phosphate solution (adjust pH to 5.3 with sodium hydroxide test solution) (70:30);
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com