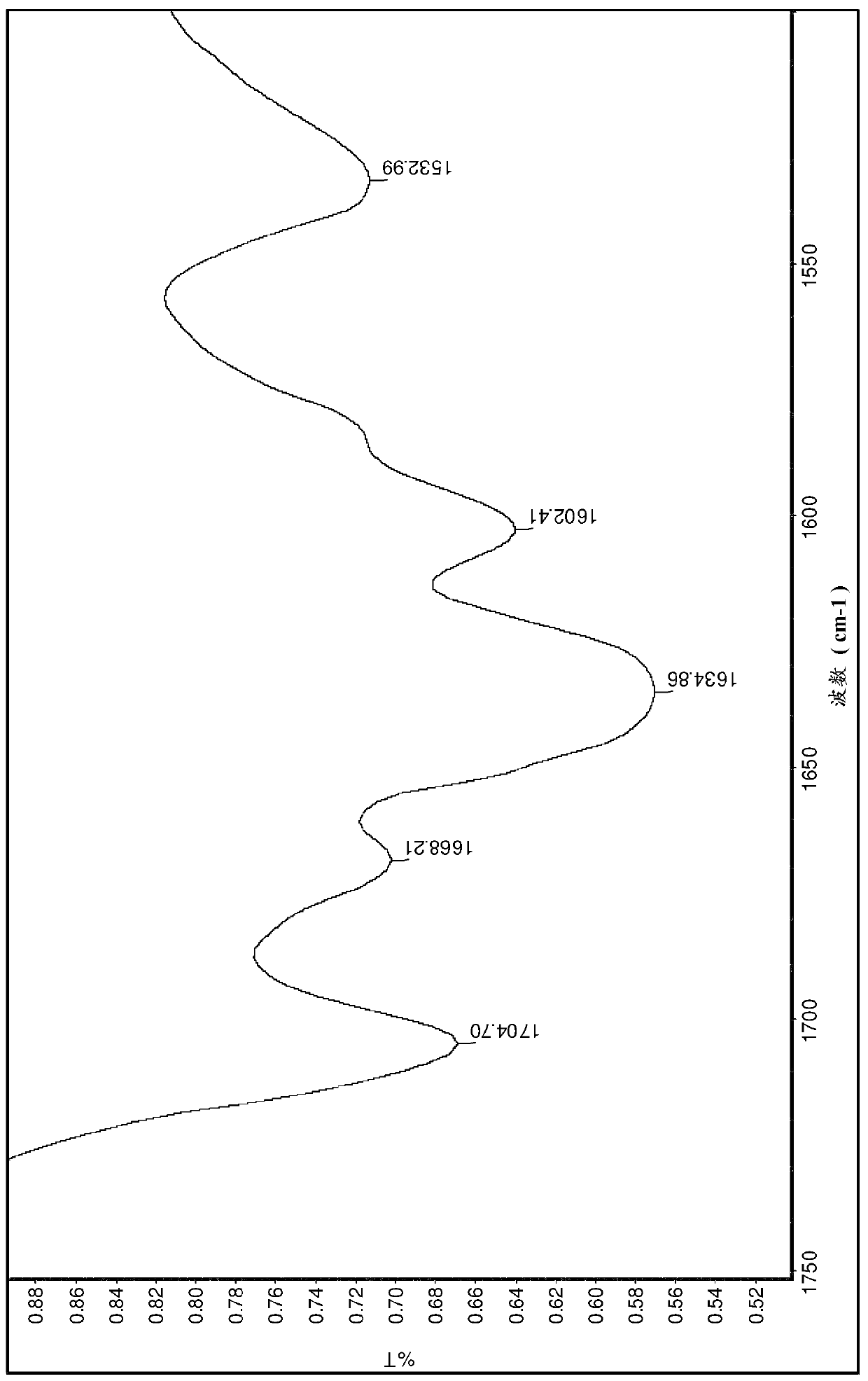
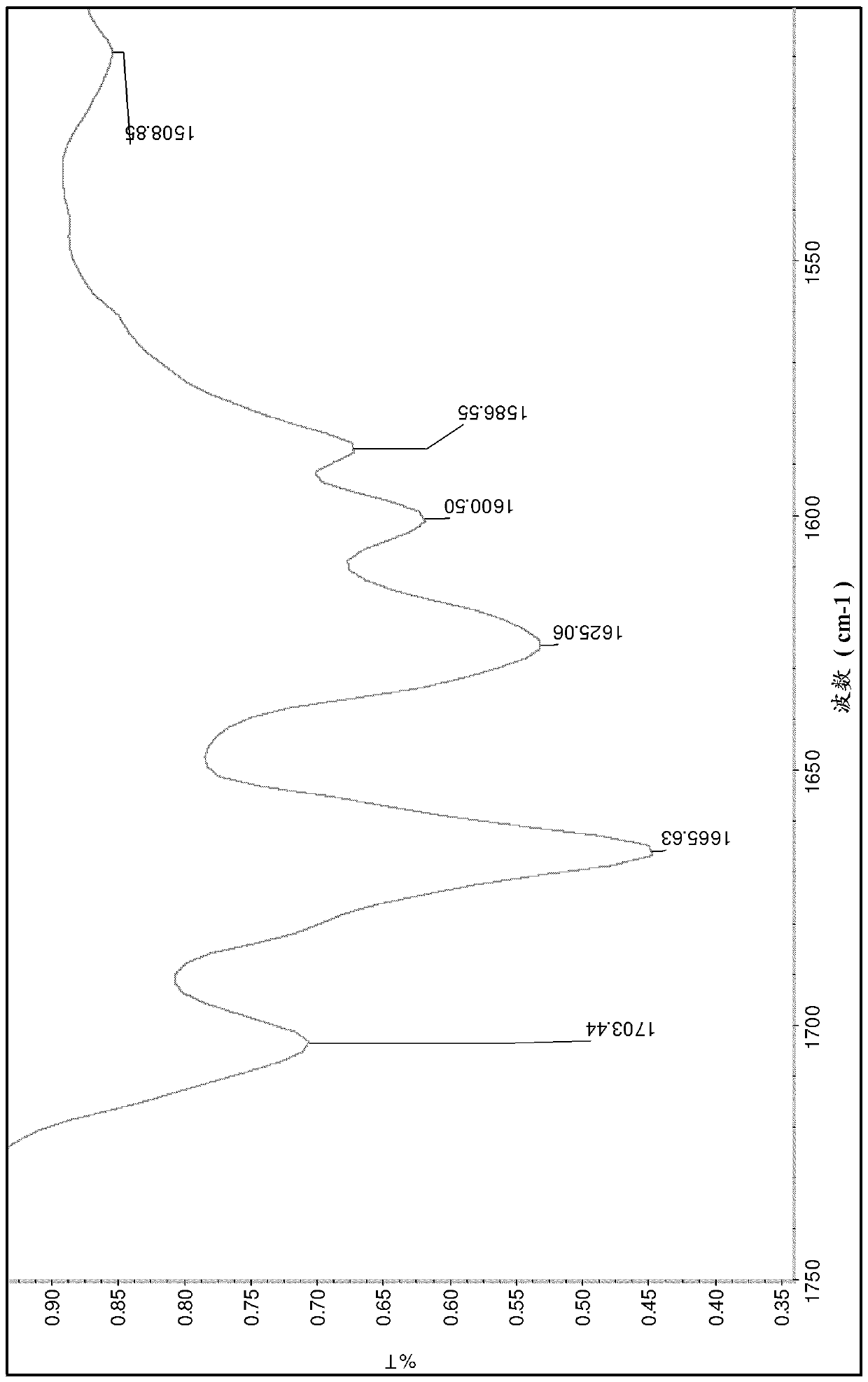
Pharmaceutical compositions comprising sepiapterin and uses thereof
A composition, the technology of mepterin, applied in the direction of drug combination, medical preparations containing active ingredients, medical preparations with non-active ingredients, etc., can solve problems such as nerve movement defects and reduce the formation of neurotransmitters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0242] Example 1. Preparation of a pharmaceutical composition comprising mepterin and an antioxidant
[0243] The process related to the manufacture of a pharmaceutical composition comprising mepterin and less than 10% by total weight of an antioxidant is as follows:
[0244] 1. Put mepterin (0.34 kg), ascorbic acid (0.071 kg), microcrystalline cellulose (0.85 kg), croscarmellose sodium (0.14 kg) and colloidal silicon dioxide (0.085 kg) separately Screen through a 140 mesh sieve.
[0245] 2. Mix colloidal silicon dioxide with microcrystalline cellulose and pass the combined material through an 80 mesh screen.
[0246] 3. Put the sifted material from step 2 into the V-blender.
[0247] 4. Add the filtered croscarmellose, ascorbic acid and mepterin to the V-blender.
[0248] 5. Blend the contents of the V-blender for at least 10 minutes.
[0249] 6. Pass the mixture through a 140-mesh sieve.
[0250] 7. Store the mixture at -20°C.
Embodiment 2
[0251] Example 2. Preparation of Pharmaceutical Compositions Comprising Mepterin and Antioxidants in an Administration Vehicle
[0252] The composition prepared in Example 1 (180 mg / kg methotrexate) was added to an amount of Medisca® oral mix (2.5% (w / w) in water) sufficient to produce a metopterin concentration of 58.3 mg / mL glycerol, 27% (w / w) sucrose).
Embodiment 3
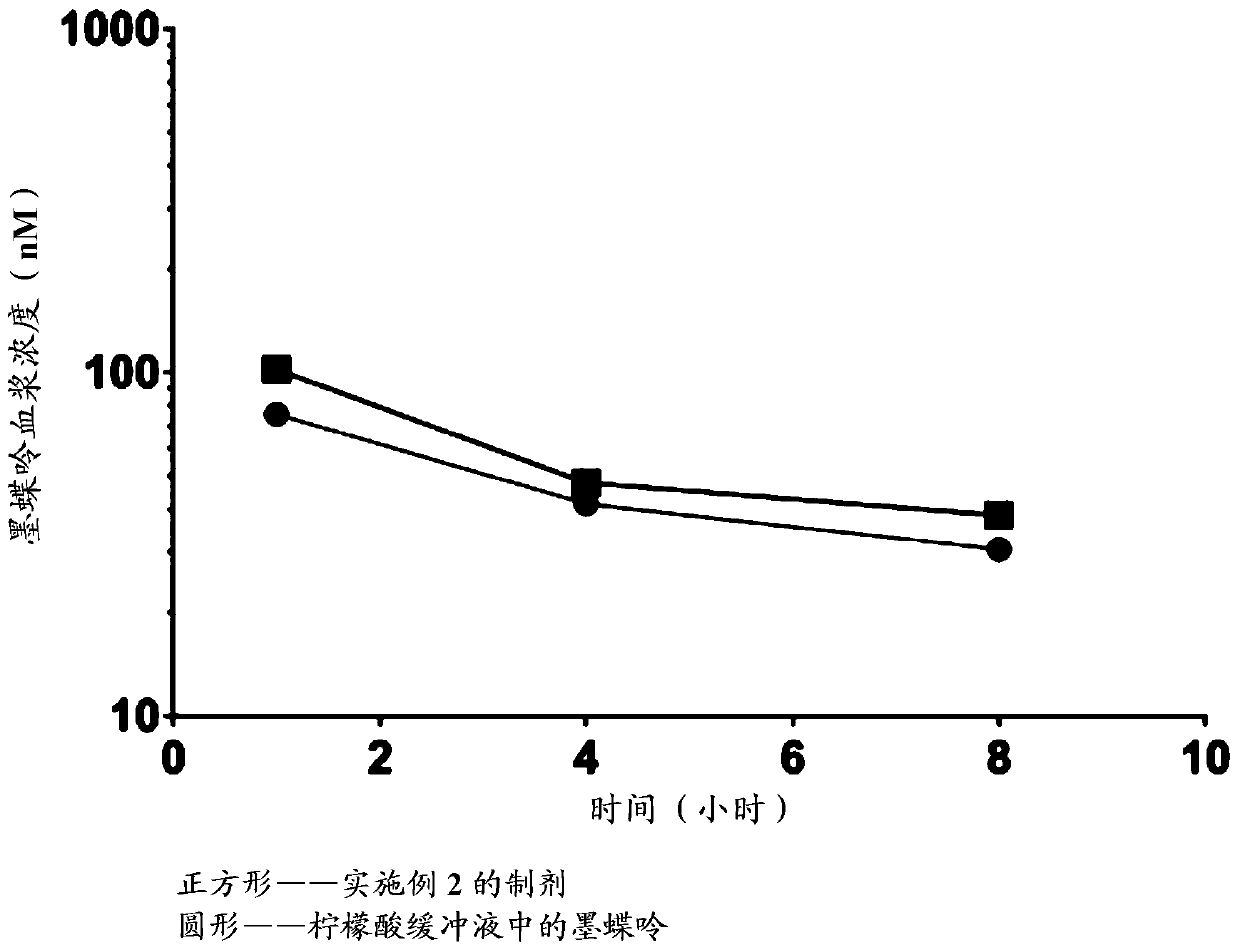
[0253] Embodiment 3. In vivo pharmacokinetic analysis of the pharmaceutical composition of the present invention
[0254] A total of 18 CD-1 mice were divided into 2 groups as summarized below. Each test article was evaluated at one dose level (180 mg / kg). All animals in each group received a single oral (PO) administration of one dose level of the test or control article. Doses (mg / kg) were calculated based on animal body weight. There were nine male mice in each group. Group 1 was given mepterin added to a citrate buffer solution consisting of 750 mg sodium metabisulfite, 639 mg sodium citrate, 2.69 g anhydrous citric acid, and 750 mg ascorbic acid in 300 mL sterile water . Then 1.5 grams of carboxymethylcellulose was added and stirred until dissolved. Mepterin (180 mg / kg) was added to the buffer to give a concentration of 27 mg / mL. Administer group 1 mice at a dose volume of 6.67 mL / kg. The preparation prepared in Example 2 was administered to the second group. Admi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com