Antibody and preparation method and application thereof
A technology of antibody and antibody library, applied in the field of biomedicine, can solve the problems of difficulty in producing high-titer high-affinity antibodies, weak immunogenicity, etc., and achieve the effect of high affinity, strong stability, and easy modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Construction of phage display lamprey-derived antibody primary library against AFB1
[0074] 1. RNA extraction and cDNA synthesis
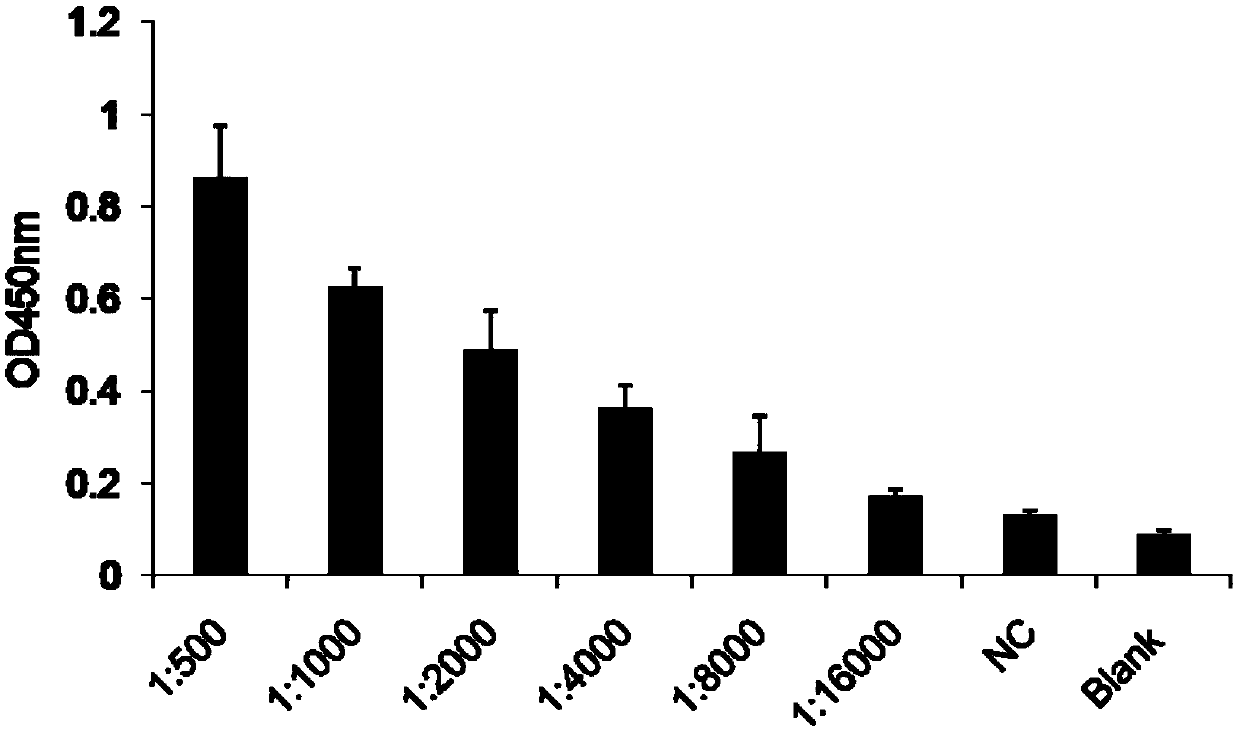
[0075] Dock the tail of lamprey immunized with complete antigen AFB1-HEL (serum titer 1:16000) to take blood, and ELISA method to detect lamprey anti-AFB1-HEL serum titer results see figure 1 , lymphocytes were isolated, the total RNA of lymphocytes was lysed and extracted with Trizol, and the first strand of cDNA was synthesized using a reverse transcription kit.
[0076] 2. Amplification of the VLR gene
[0077] 1. Primer design:
[0078] Primer F (including Nco I restriction site) SEQ ID NO.5: CATGCCATGGGTTGGATCAAGTGGATCGCCACG
[0079] Primer R (including Not I restriction site) SEQ ID NO.6: ATAAGAATGCGGCCGCACGTTTCTTGCAGAGGGCG
[0080] 2. PCR amplification system of VLR gene fragments:
[0081] Lamprey leukocyte cDNA was used as a template and amplified with Pyrobest DNA polymerase. The PCR amplification system is shown in...
Embodiment 2
[0086] Construction and panning of the phage antibody library of embodiment 2
[0087] 1. Connection between VLR and pCANTAB-5E
[0088] Follow the steps in Table 2 below:
[0089] Table 2
[0090]
[0091]
[0092] Ligate at 16°C for 12-16 hours, and extinguish T4 ligase at 70°C for 10 minutes.
[0093] 2. Construction of VLR library - electrotransformation of recombinant plasmid pCANTAB 5E-VLR
[0094] 1) Melt the recombinant plasmid pCANTAB 5E-VLR and electroporation competent TG1 on ice, and put the electroporation cup on ice to pre-cool;
[0095] 2) Take 4 μL of the recombinant plasmid pCANTAB 5E-VLR and add it to the melted competent TG1, and gently pipette to mix;
[0096] 3) Add the competent cells mixed with the recombinant plasmid into the pre-cooled 0.1cm electroporation cup, cover the lid, and gently tap the bottom of the cup on the table, so that the competent cells are evenly distributed on the bottom of the cup, and at the same time exclude Bubbles, q...
Embodiment 3
[0134] Example 3 Detection of positive phage single-chain antibody function
[0135] 1. Phage ELISA detection of positive clones
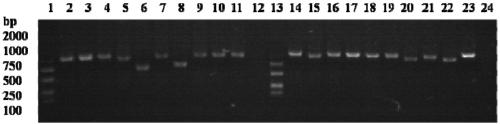
[0136] The present invention randomly picks 94 single clones from the 10cm plate whose titer is measured after the fifth round of screening, infects with helper phage, displays VLR on the capsid protein of the phage, and then coats the microtiter plate with AFB1-BSA , carry out phage ELISA to screen anti-AFB1 recombinant phage; the last two wells (95, 96) use helper phage as negative control wells, and the OD450 is 0.046 and 0.047 respectively. From the results of ELISA, it can be seen that there are many clones with higher affinity in the 94 clones. clone (results see Figure 5 );
[0137] 1) Mix 40 μg of protein antigen AFB1-BSA in 20 mL of PBS and coat a 94-well microtiter plate.
[0138] Well 100μL, put in 4 ℃ refrigerator overnight, you can also use carbonate buffer;
[0139] 2) Discard the antigen coating solution, and wash 3 times with t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com