Method for determining dimethyl sulfate in medicine by derivatization gas chromatography-mass spectrometry
A technology of dimethyl sulfate and gas chromatography, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems affecting the specificity, accuracy, and long derivation reaction time of the detection, and achieve accurate and reliable detection results, specificity and Good sensitivity, simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The mensuration of dimethyl sulfate content in embodiment 1, antiulcer medicine
[0044] 1. Chromatographic conditions
[0045] Instrument: Agilent 7890B Gas Chromatograph-5977B Mass Spectrometer-CTC Autosampler
[0046] Chromatographic column: VF-624ms (30m×0.25mm×1.4μm)
[0047] Inlet temperature: 200°C
[0048] Injection volume: 1ml
[0049] Split ratio: 50:1
[0050] Carrier gas: helium (constant flow 1.0mL / min)
[0051] Headspace conditions: equilibrium temperature 60°C, equilibrium time 30min
[0052] Column temperature: keep at 40°C for 2min, raise the temperature to 100°C at 10°C / min, then raise the temperature to 220°C at 30°C / min, and keep for 3min.
[0053] Detector: mass spectrometer detector, ion source temperature 230°C, quadrupole temperature 150°C
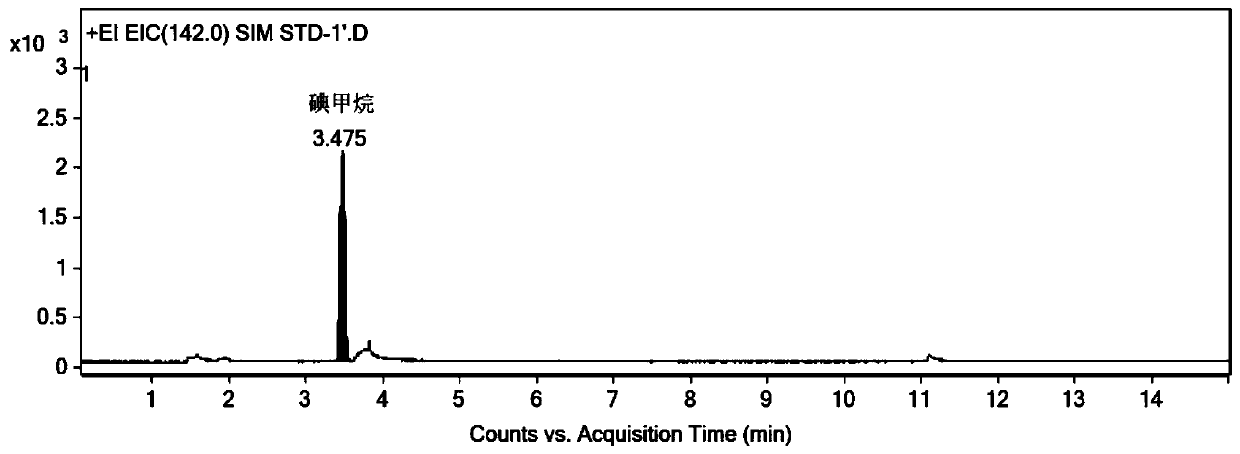
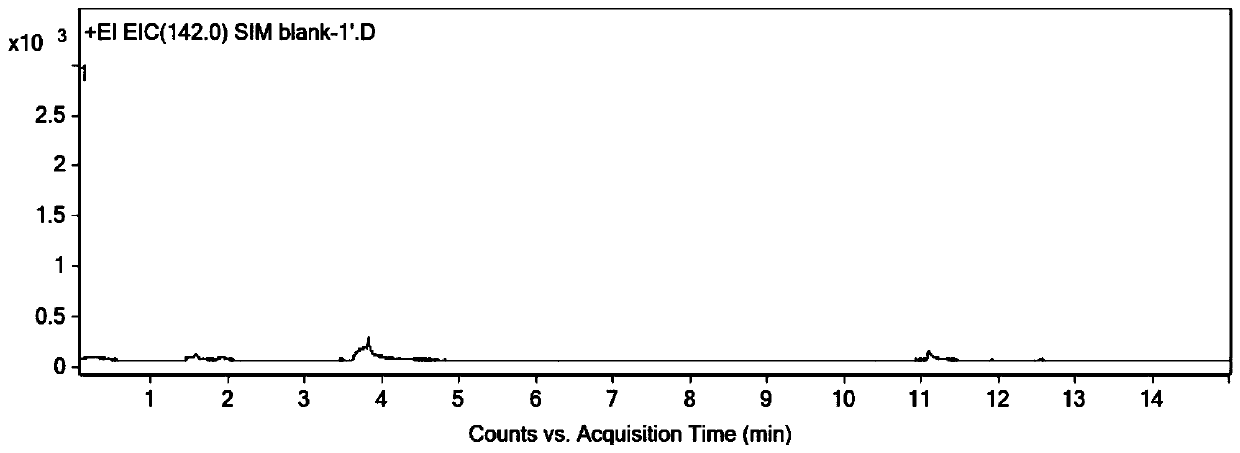
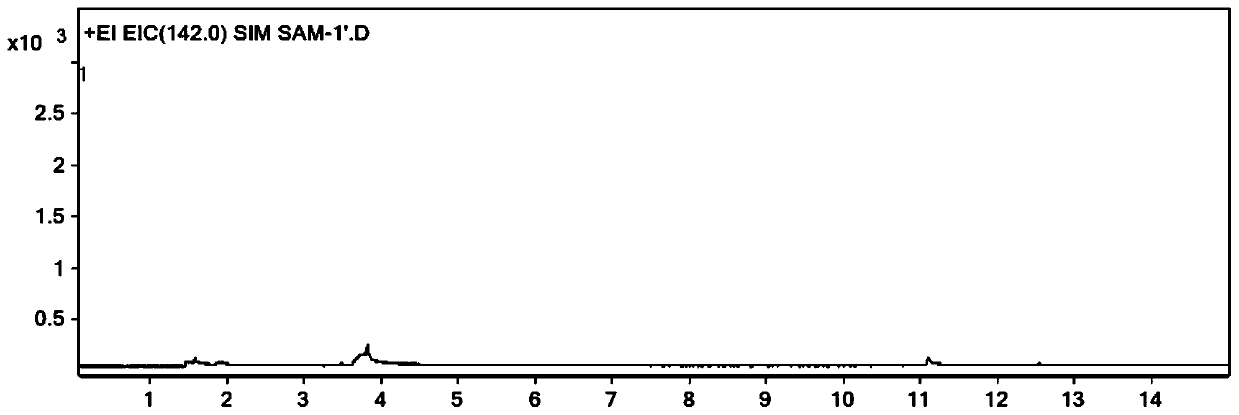
[0054] Selected ion monitoring mode (127, 142): m / z127 is used as an auxiliary qualitative ion, and m / z142 is used as a quantitative ion of dimethyl sulfate derivatized product methyl iodide.
[0055]...
Embodiment 2
[0062] Embodiment 2, the method verification of chromatographic method
[0063] 1. Exclusiveness
[0064] Precisely pipette 1ml of acetonitrile and 1ml of derivatization solution into a 20ml headspace bottle, seal it, shake well, and use it as a blank solution. Accurately weigh an appropriate amount of dimethyl sulfate, add acetonitrile to dilute to make a solution containing about 2 mg of dimethyl sulfate per 1 ml, as a stock solution; dilute the stock solution step by step with acetonitrile to obtain dimethyl sulfate with a concentration of 100 ng / mL Ester solution; pipette 1mL dimethyl sulfate solution and 1mL derivatization solution into a 20mL headspace bottle, seal and shake well to obtain a control solution with a concentration of 50ng / mL. Accurately weigh about 0.04g of the sample to be tested (ranitidine hydrochloride) into a 20mL headspace bottle, add 1mL of acetonitrile and 1mL of derivatization solution, seal, shake well, and obtain the test solution. Accuratel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com