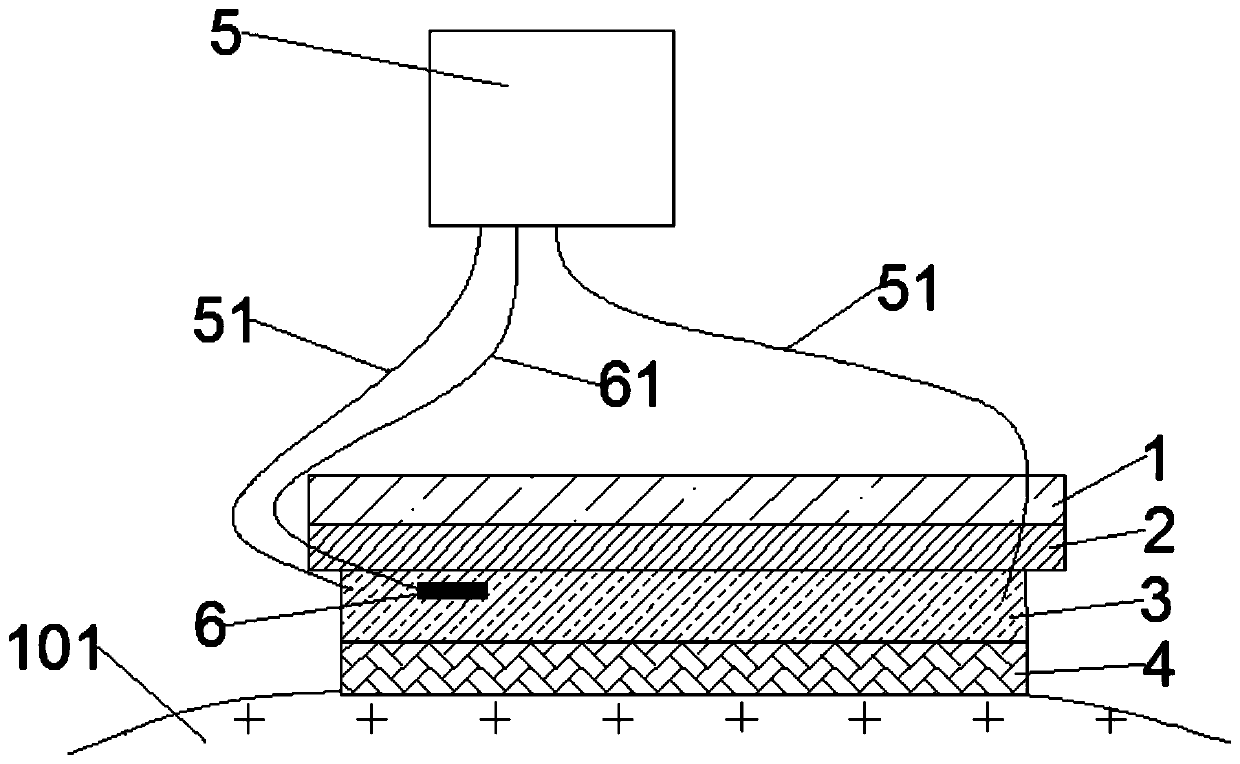
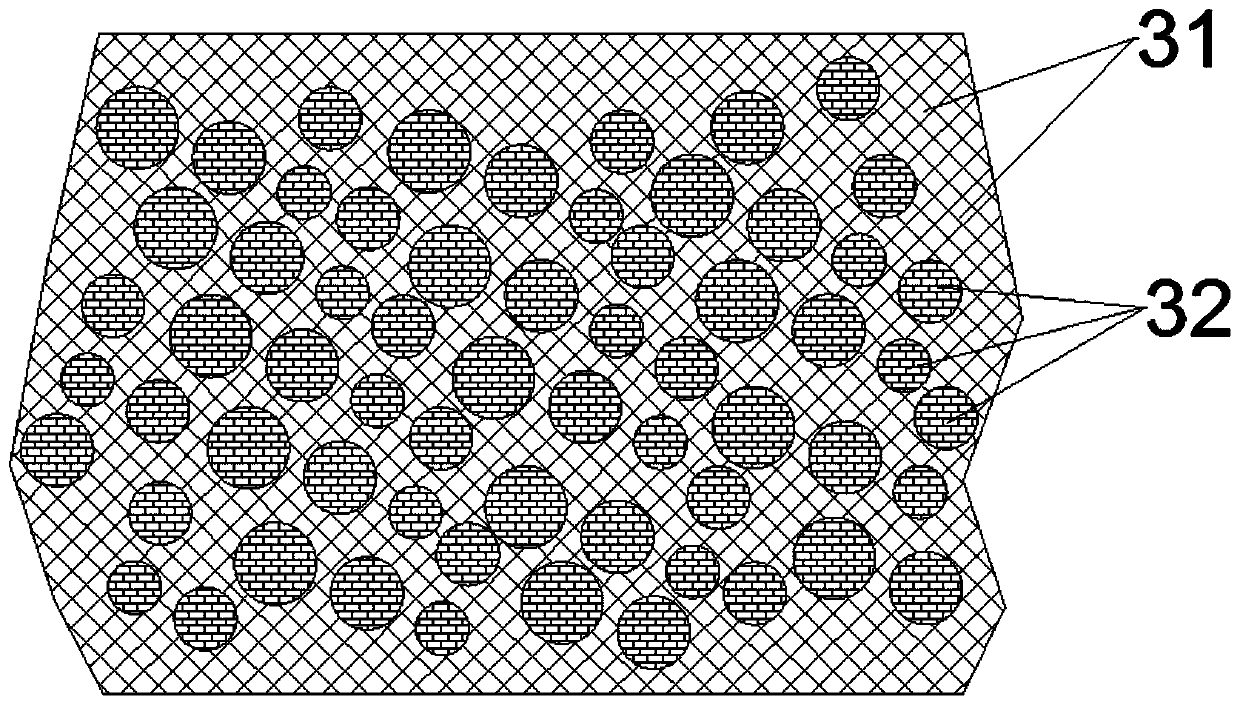
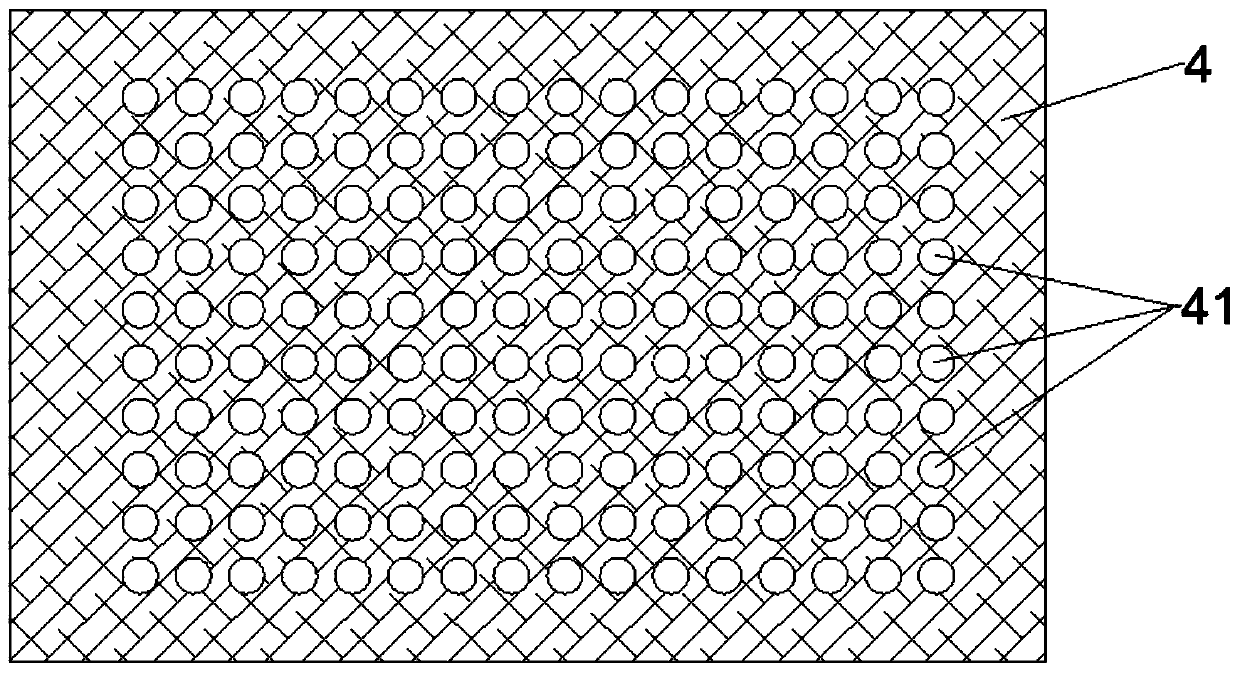
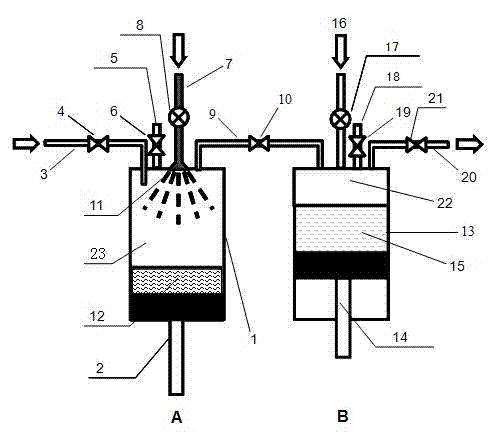
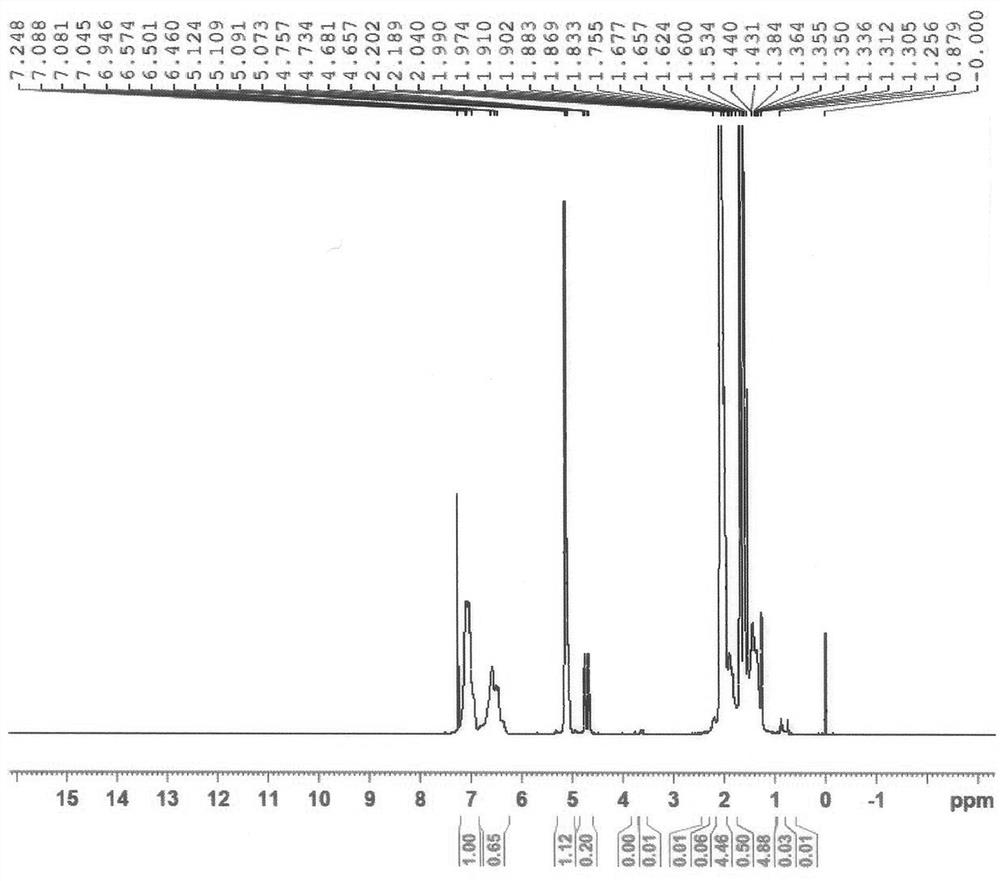
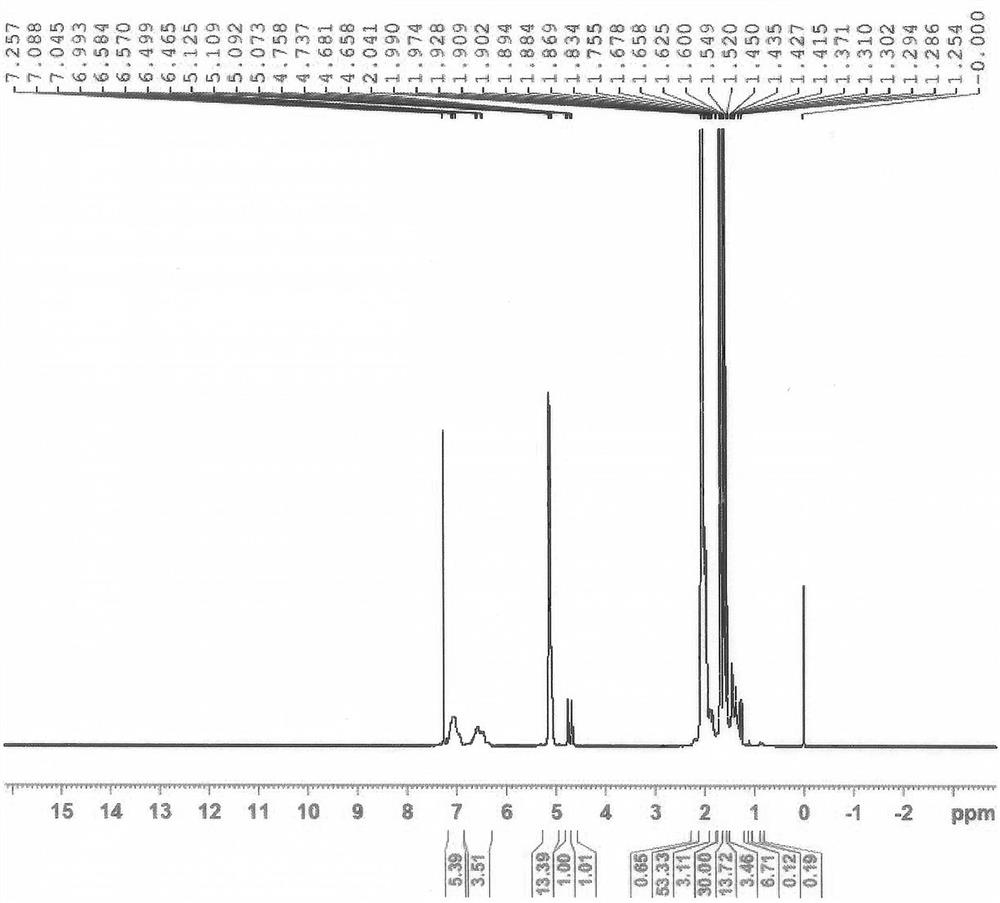
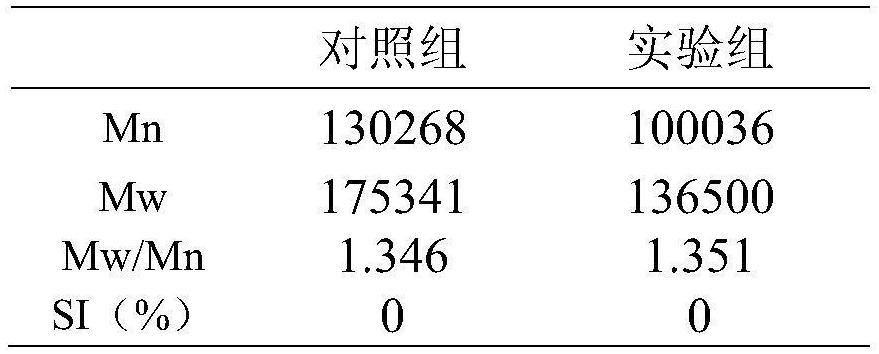
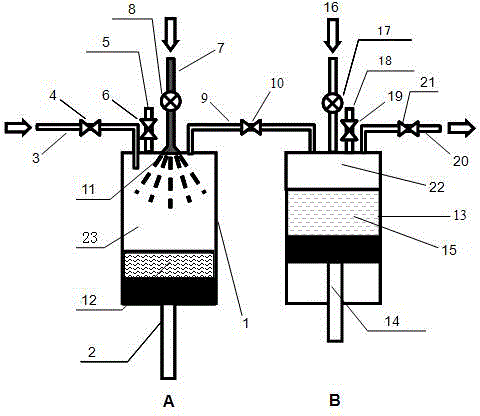
Patents
Literature
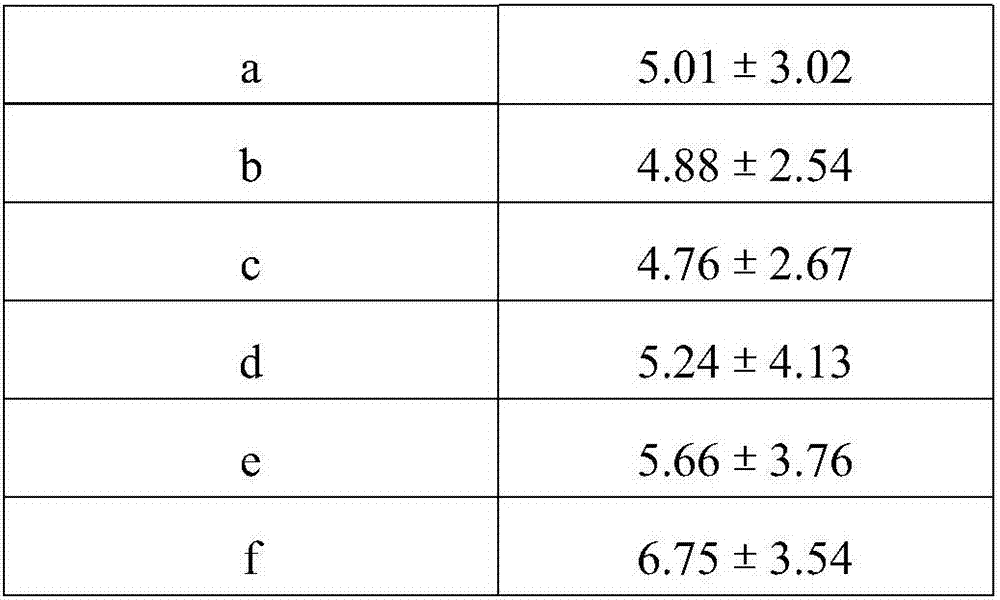
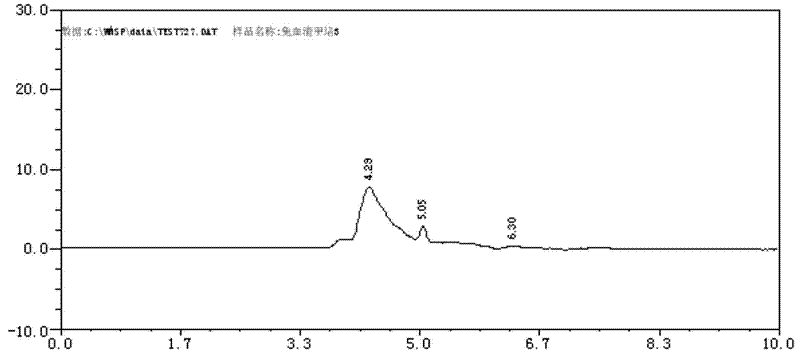
36 results about "Volatile drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Volatile substances drug profile. Domestic products such as spray deodorants, glue, lighter refills and spray air fresheners can be used as drugs. Volatile substance use may be defined as the deliberate inhalation of volatile compounds to produce psychoactive effects.
Microencapsulation method of Chinese traditional medicine
InactiveCN1593504AFix stability issuesSolve environmental problemsUnknown materialsMicrocapsulesCross-linkMicrosphere
The invention relates to a coated medicine-carrying process for volatile medicament or oil form traditional Chinese medicine with mucosity such as borneol, which consists of dissolving the medicinal components and capsule material in organic solvent, stirring and charging into the aqueous phase of surface active agent, then performing cross-linking, aging, filtering and drying, thus obtaining coated medicine-carrying microcapsule or microsphere.
Owner:TIANJIN UNIV
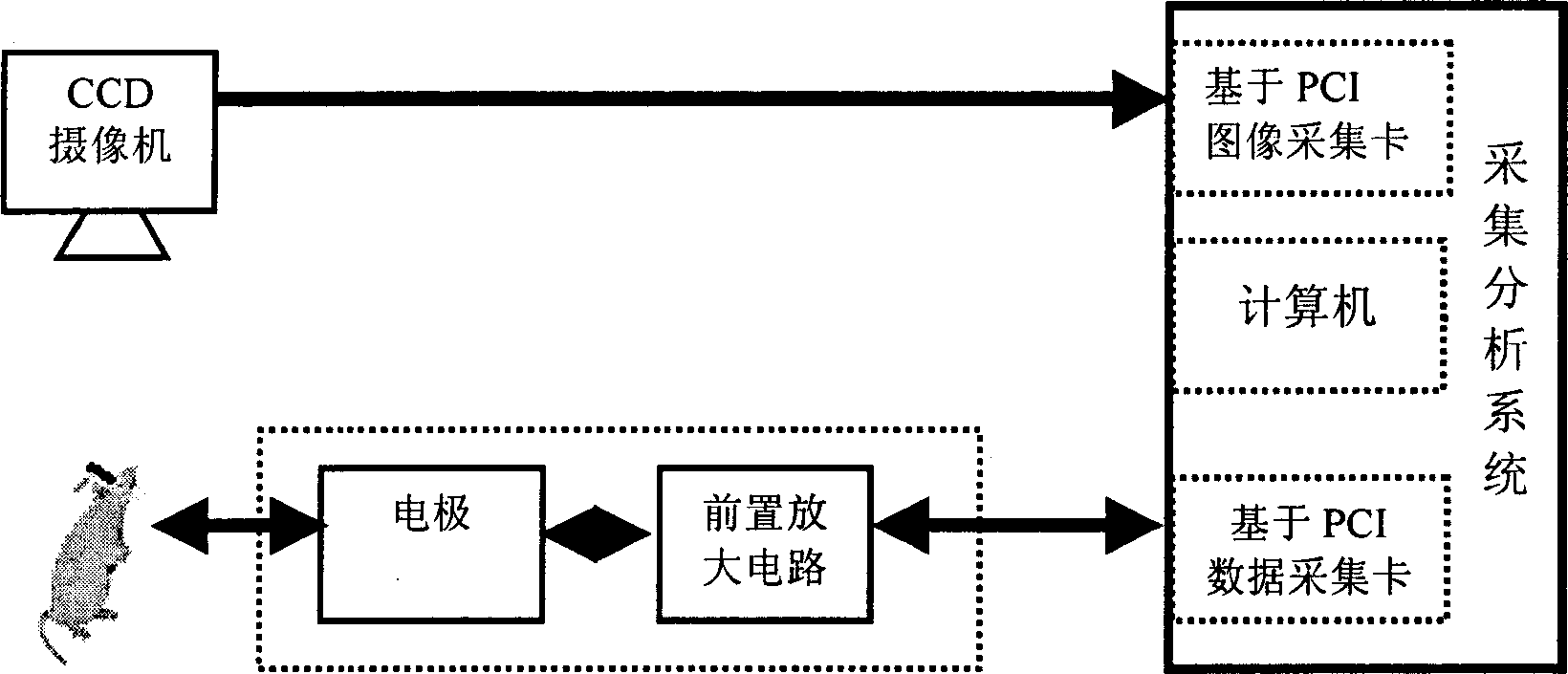
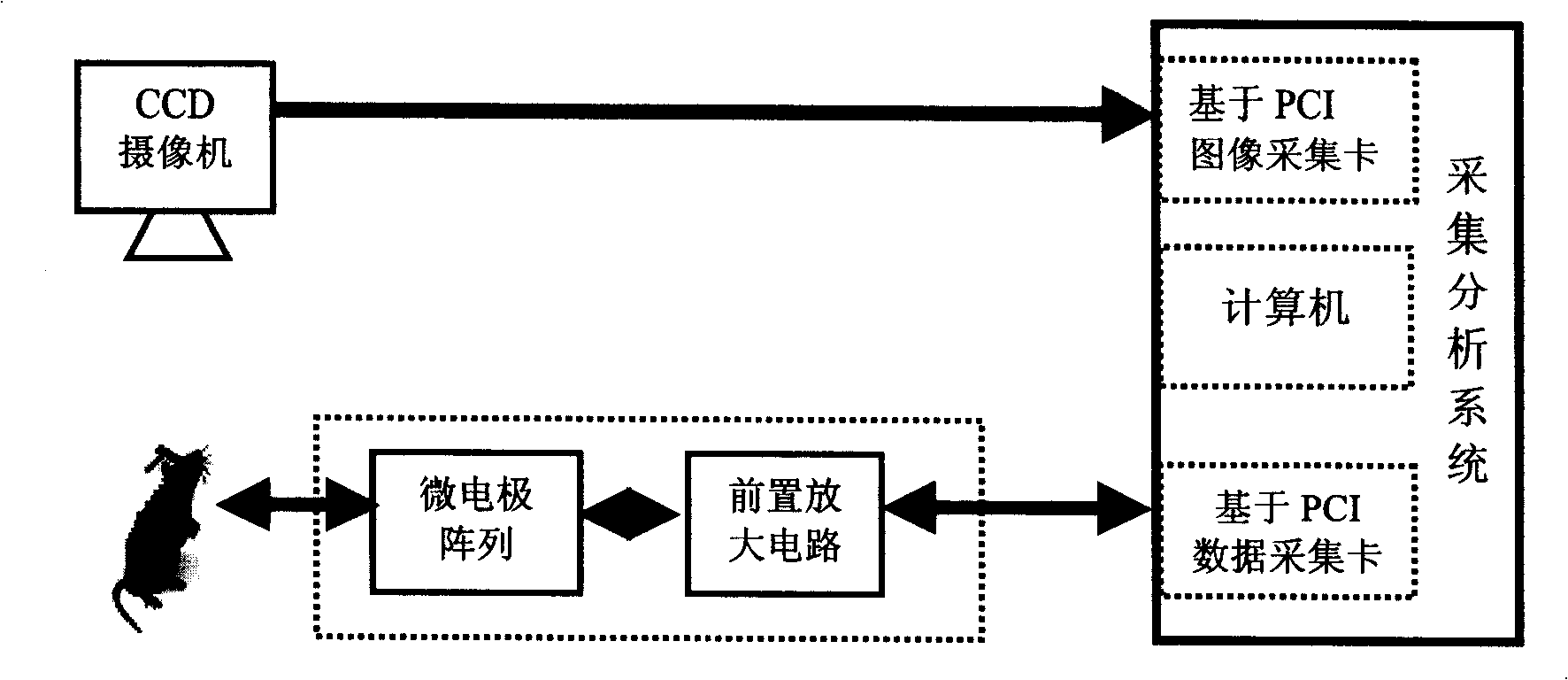
Drug detector based on animal olfactory sensation
InactiveCN1865996ASolve the characteristicsResolve detectionDiagnostic recording/measuringSensorsAnimal brainEngineering
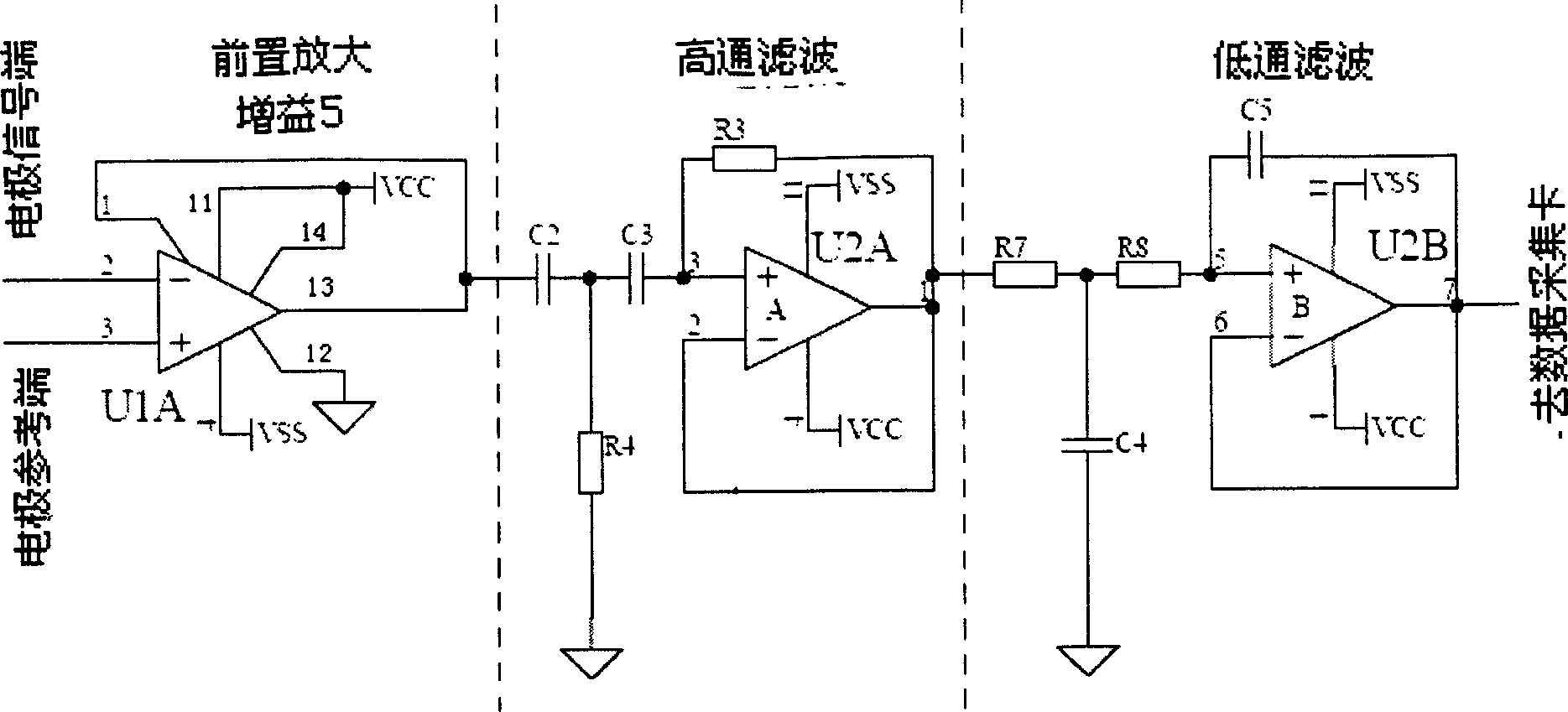
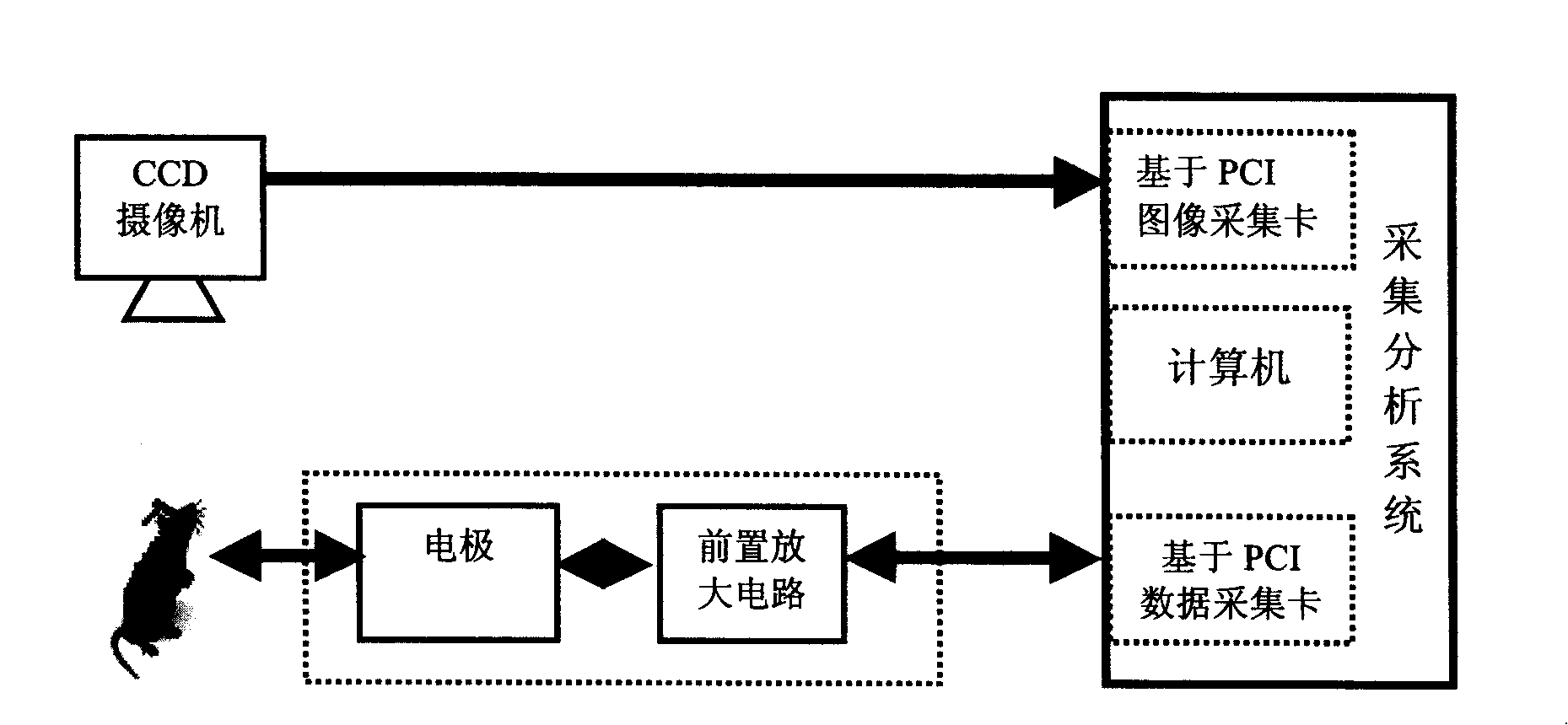
The disclosed drug detector based on animal olfaction comprises: a CCD camera, a brain stereotaxic instrument, an electrode, a pre-amplification and filter circuit, a multiarm maze, and computer control and analysis system. Wherein, according to animal brain olfaction cortex neuron electric action, letting animal produce specific memory to volatile drug; random putting drug in small rooms of the maze to give prize stimulation for specific brain area and reinforce the memory till animal action includes statistic meaning, collecting relative induced brain electric information to input computer for recognition and analysis and form training database.
Owner:XI AN JIAOTONG UNIV
Volatile medicine with eye health-care effect
InactiveCN105687296AMild health careAbsorb enoughSenses disorderHydroxy compound active ingredientsJojoba oilVolatile drugs
The invention provides a volatile medicine with an eye health-care effect. The medicine is prepared from one or more of d-borneol essential oil, lavender essential oil, chamomile essential oil, calendula officinalis essential oil, rosemary essential oil, peppermint essential oil, grapefruit essential oil, benzoin essential oil, rose essential oil, geranium essential oil, jojoba oil, dalbergia odorifera essential oil, rose hip essential oil, frankincense essential oil, evening primrose essential oil, sandalwood essential oil, radix bupleuri essential oil, chrysanthemum essential oil, wolfberry essential oil, cassia seed essential oil, lemon essential oil, pomegranate seed essential oil, safflower essential oil and natural vitamin E. The volatile medicine is prepared from natural volatile medicinal materials, eyes are surrounded in the atmosphere formed after the medicinal materials are volatized, and the medicinal materials are slowly and mildly released. The volatile medicine can be applied to daily eye health care and is used together with an eye therapeutic apparatus.
Owner:HANGZHOU LUKE TECH CO LTD
Multifunctional health care medical pillow for children
InactiveCN101185787AReasonable designStructural sciencePillowsAnthropod material medical ingredientsDiseaseVolatile drugs
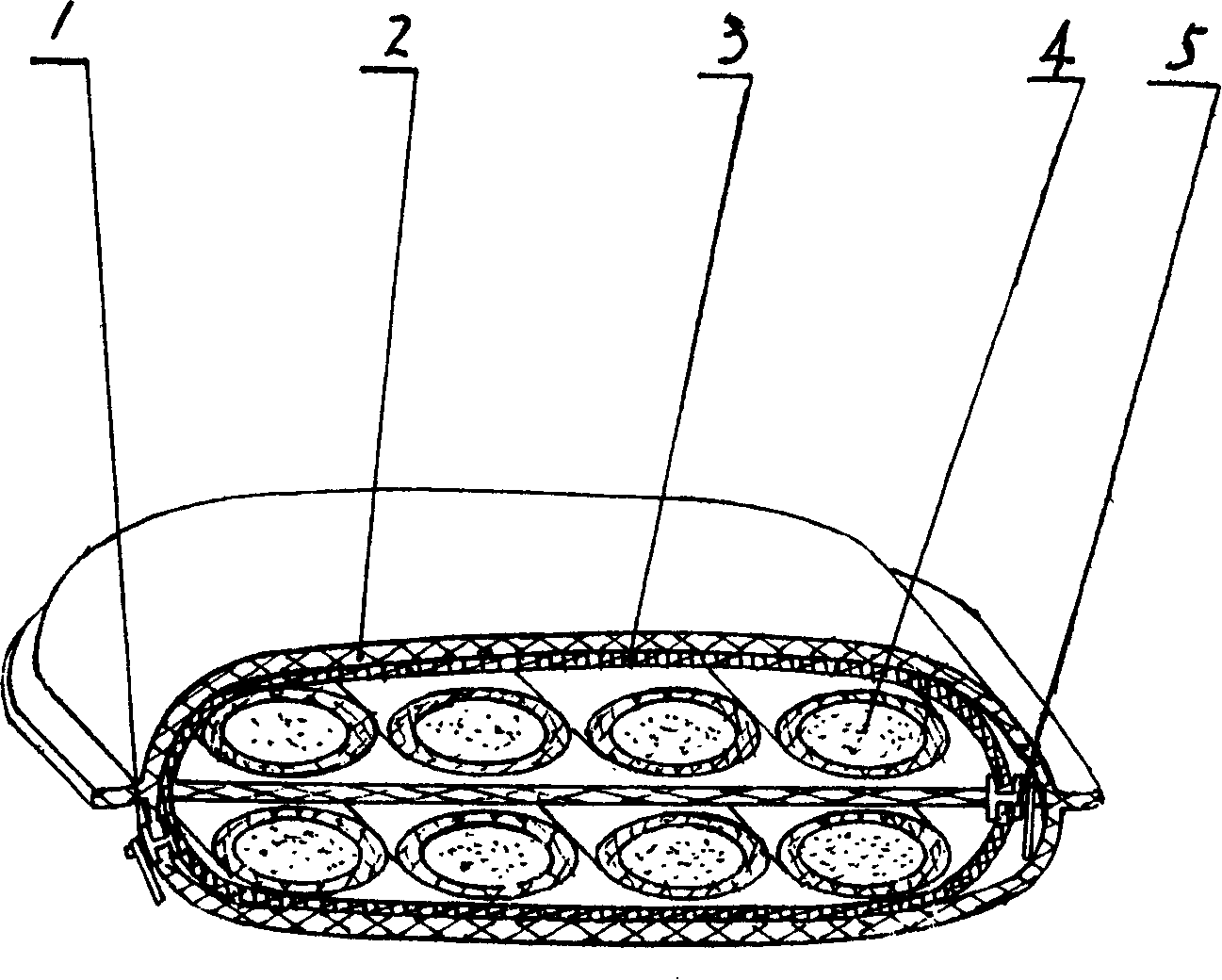
The invention relates to a multi-functional health care drug pillow for children, which has the health care function for children. The technical proposal is that: the multi-functional health care drug pillow for children is provided with a pillow outer sleeve; the invention is characterized in that: the interior of the pillow outer sleeve is provided with an inner cover, the interior of the inner cover is provided with a separation layer, and four drug pillow cores are respectively arranged above and below the separation layer. The positions of the drug pillow cores can be adjusted at will at any time according to different diseases, different symptoms and general health status of children. Most of the drugs in the drug pillow cores are characterized by strong drug smell, so the volatile drug smell can be easily absorbed via the respiration of people, further to achieve the functions of prevention, health care and treatment.
Owner:宋维范
Local high-pressure oxygen therapy apparatus
ActiveCN106726398APromote hyperplasiaPromote generationRespiratorsBreathing protectionMassageHeating effect
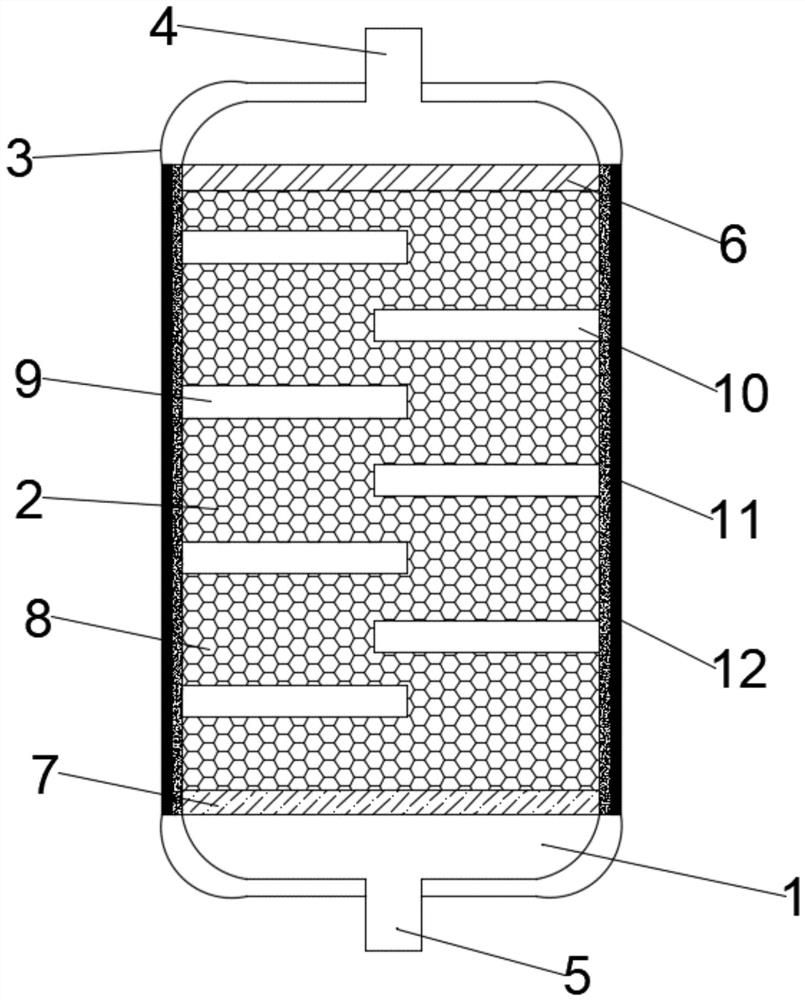
The invention discloses a local high-pressure oxygen therapy apparatus which comprises a main air bag, an oxygen generating device, a massage device, a heating sheet and a treatment unit, wherein a cavity with an opening is arranged at one end of the main air bag, and a seal part is arranged in the opening; the oxygen generating device comprises an oxygen generator and a first pressure control mechanism, the oxygen generator is used for generating oxygen, and an oxygen outlet of the oxygen generator communicates with the inside of the main air bag through a first air inlet pipe and introduces oxygen into the main air bag; the first pressure control mechanism controls the air inflation quantity of the oxygen generator on the main air bag and keeps a gas pressure value in the main air bag within a range of 1-10KPa; the heating sheet is arranged in the main air bag and generates heat; and the treatment unit is detachably arranged in the position which is in the main air bag and corresponds to the heating sheet, and the treatment unit contains volatile drug ingredients under the heating effect.
Owner:BAODING BAIENJIE BIOTECH CO LTD
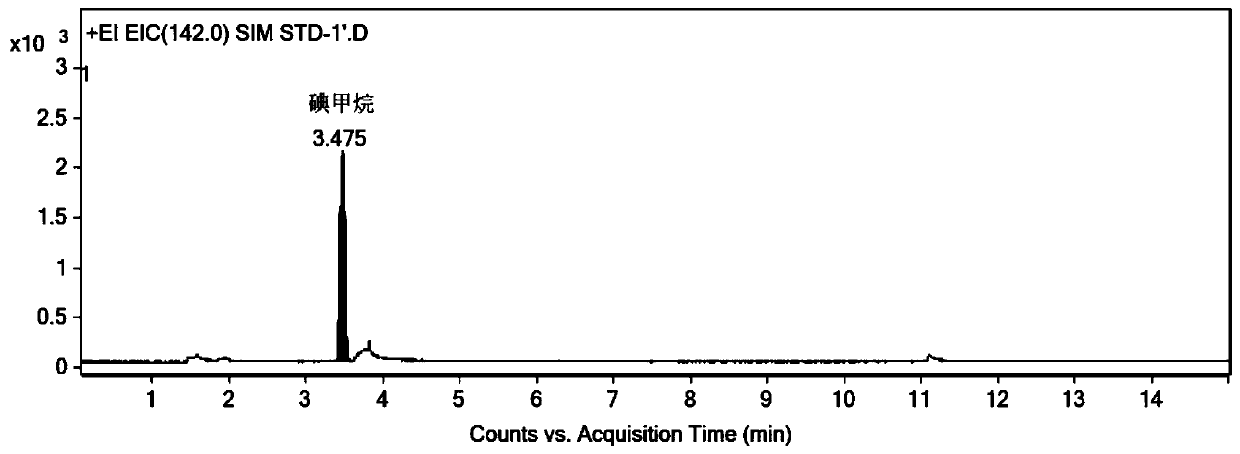
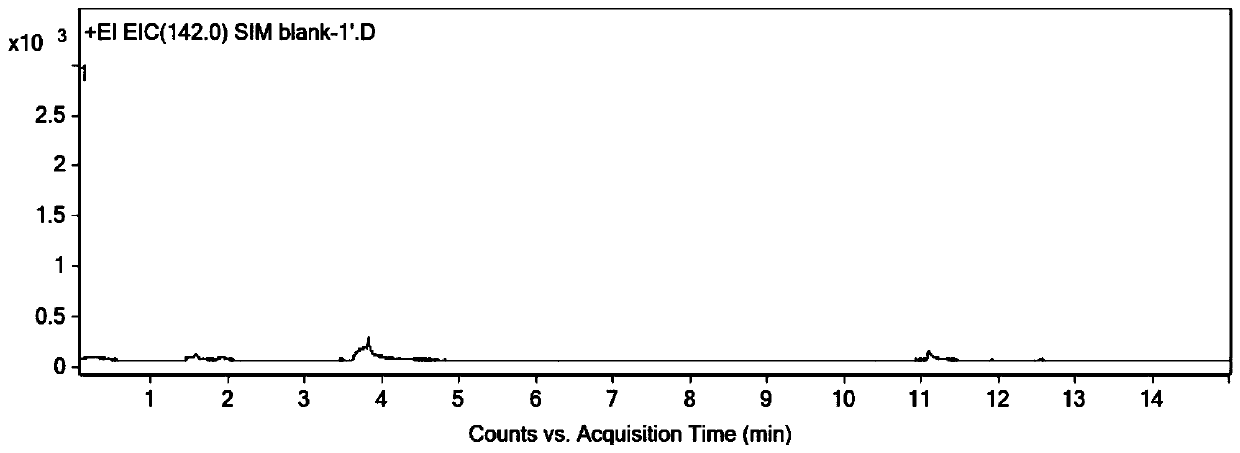
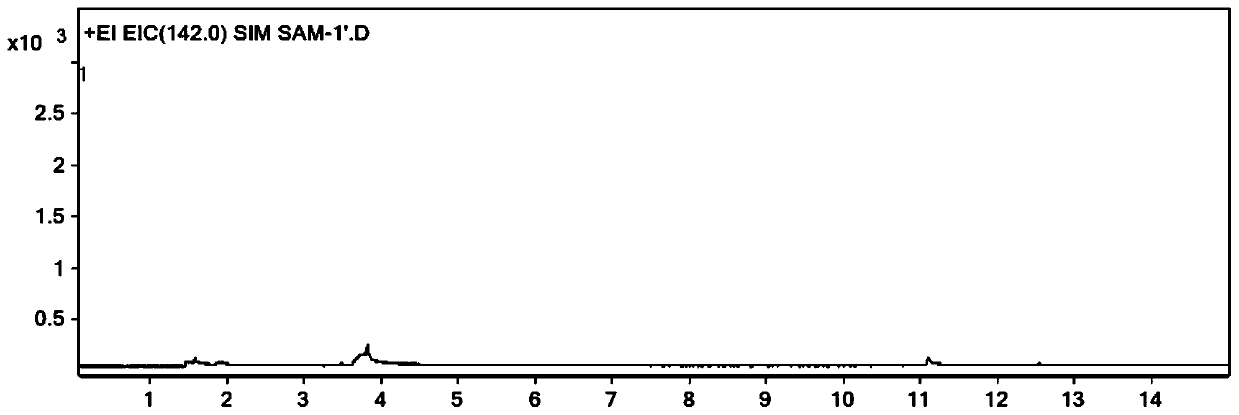
Method for determining dimethyl sulfate in medicine by derivatization gas chromatography-mass spectrometry
InactiveCN111505182AShorten the timeEasy to operateComponent separationGas liquid chromatographicSodium iodide
The invention discloses a method for determining dimethyl sulfate in a drug through derivatization gas chromatography-mass spectrometry. The method comprises the following steps of adding a derivatization solution into a drug sample, deriving dimethyl sulfate into methyl iodide, and detecting the content of methyl iodide through gas chromatography-mass spectrometry to obtain the content of dimethyl sulfate, wherein the derivatization solution is a saturated sodium iodide aqueous solution added with a trace amount of sodium thiosulfate. By adopting the method, the content of the genotoxic impurity dimethyl sulfate in the medicine can be accurately determined, and the method has the advantages of being convenient to operate, being capable of reducing the interference of a non-volatile medicine matrix and the like.
Owner:SHANGHAI SCIENPHARM CO LTD
Medicinal vapor filtration system
ActiveUS10376657B2Reduce cross contaminationMinimizes and prevents cross-contaminationTobacco smoke filtersTobacco pipesParticulatesPolyolefin
A system for filtering vaporized medicinal compounds includes a vaporizer configured to release one or more volatilized medicinal compounds in gaseous form, together with solid or semi-solid particulate byproducts, and one or more removable filters for efficiently removing at least a portion of the particulate byproducts while allowing passage of medicinally active volatilized compounds. The vaporizer includes a mouthpiece through which one or more volatilized medicinal compounds may be drawn. The filter is made of a chemically inert material (e.g., polypropylene or other polyolefin) and may be configured to detachably interface with an exposed surface of a vaporizer mouthpiece. The filter acts to remove solid or semi-solid particulate matter passing through the mouthpiece with minimal chemical and / or physical interference with volatilized medicinal compounds, which pass through the filter. The removable filter can also prevent cross-contamination by multiple users of the vaporizer.
Owner:JONES BRENDAN
Chimonanthus nitense leaf drop pills for preventing common cold and preparation technique thereof
The invention discloses a Chimonanthus nitense leaf drop pills for preventing common cold and preparation process, wherein the drop piils comprises (by weight ratio) 1,8cineole 1-10 parts and flavonoid glycoside 1-10 parts extracted from Chimonanthus intense leaves, water-based or oil-based base material 7-150 parts, excipient 0.01-10 parts.
Owner:江西佑美制药有限公司
Infectious disease prevention and control mask with disinfection effect
InactiveCN111296932ASuitable for prevention and controlImprove the blocking effectSynthetic polymeric active ingredientsRespiratory disorderPathogenic microorganismInfectious Disorder
The invention relates to an infectious disease prevention and control mask with a disinfection effect. The prevention and control mask comprises a plush protective mask which is integrally or locallyprovided with plush and can be used as a drug carrier without affecting breathing, and a medicament which can be attached to the surface of the plush, has adsorbability, can kill pathogenic bacteria and viruses, does not contain volatile drugs harmful to a human body, and does not have irritation and unpleasant odor. The prevention and control mask can play a better role in preventing inhaled tinydust, particularly inhaled and exhaled droplet pathogenic microorganisms, can kill the inhaled and exhaled droplet pathogenic microorganisms, and does not influence breathing smoothness after being worn. Besides, the mask can be worn for a long time, has the technical basis of repeated use, and is easy to treat after being discarded. The mask is more suitable for prevention and control of epidemic infectious diseases.
Owner:优仕康生(天津)科技发展有限公司
Traumatic rheumatism plaster and preparation method thereof
ActiveCN107184928AHigh extraction rateReduce energy consumptionHydroxy compound active ingredientsAntipyreticRheumatismTherapeutic effect
The invention discloses a traumatic rheumatism plaster which comprises an ointment and a medicinal plaster matrix. The ointment comprises traumatic rheumatism liquid extract which is prepared from flos carthami, radix saposhnikoviae, rhizoma zingiberis, unprocessed radix aconiti kusnezoffii, herba schizonepetae, unprocessed radix aconiti, radix angelicae, herba glechomae, rhizoma kaempferiae and unprocessed semen strychni. Volatile oil is extracted from medicine by adopting supercritical CO2 extraction technology in the preparation process, so that volatile oil extraction rate is increased, and damage to active ingredients is reduced; a volatile medicine inclusion compound is prepared by adopting inclusion technology, so that loss of volatile active ingredients in the process of preparing and storing a musk traumatic rheumatism plaster is reduced, and medicine efficacy is improved; a novel hydrophilic matrix is adopted, so that environment pollution caused by an organic solvent is avoided, allergic reaction which is prone to appearing in the process of medication is reduced, medication compliance of patients is improved, and the traumatic rheumatism plaster has remarkable treatment effect on rheumatism, swelling and joint pain.
Owner:GUANGZHOU BAIYUNSHAN JINGXIUTANG PHARM CO LTD
Microencapsulation method of Chinese traditional medicine
Owner:TIANJIN UNIV
Adjustable volatile cell for ocular surface medication
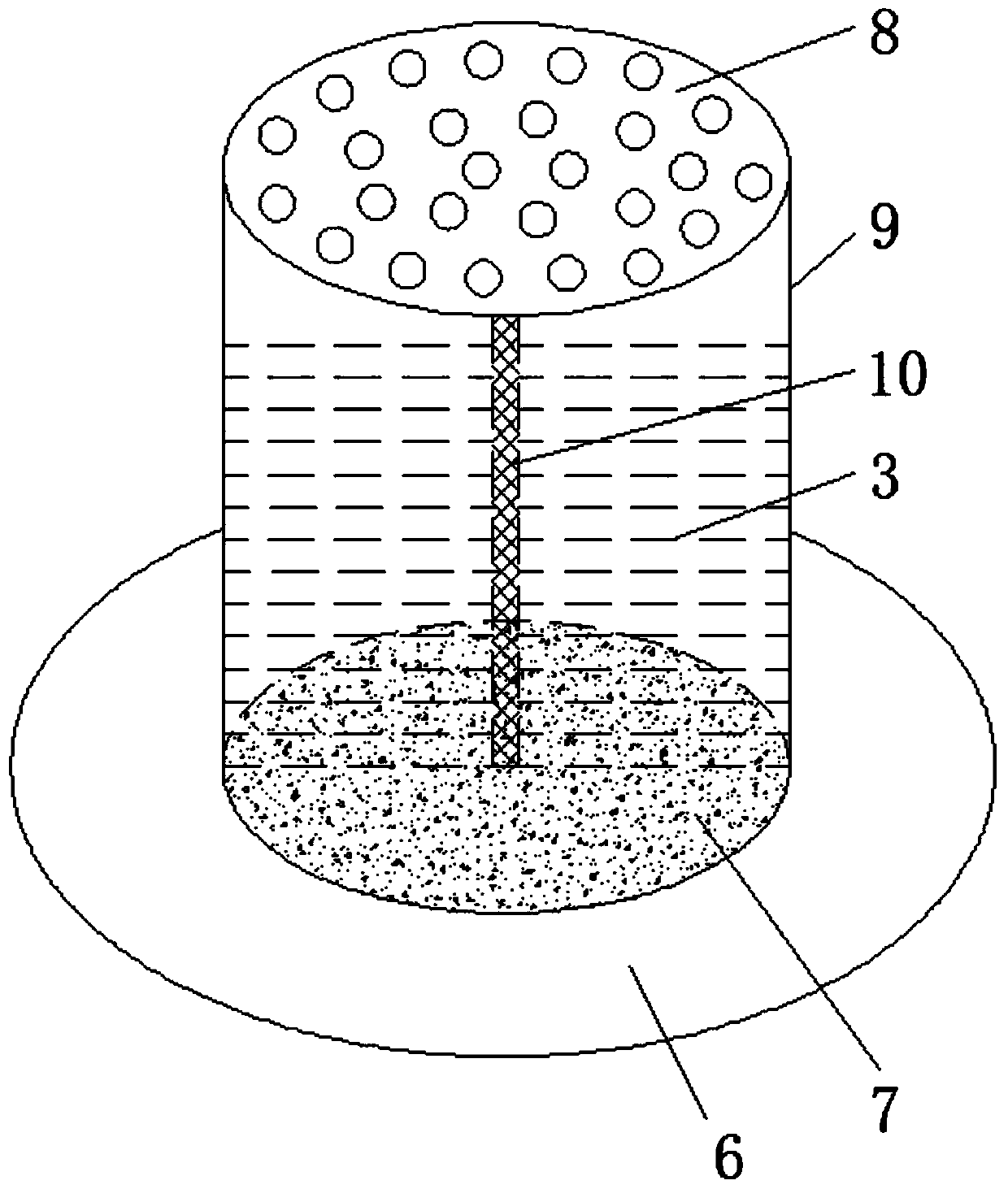
InactiveCN102871794APrevent volatilizationPrevent fallingMedical devicesEye treatmentXerophthalmiaPathogenic microorganism
The invention provides an adjustable volatile cell for ocular surface medication. The adjustable volatile cell comprises an outer frame with a groove, shaft levers, a twisting wheel and a medicine container. The medicine container is disposed in the groove. The shaft levers are connected to two ends of the medicine container. The medicine container is rotatably connected with the outer frame through the shaft levers. One of the shaft levers is connected with the twisting wheel through the outer frame. When used, the adjustable volatile cell is worn below an eye of a patient and is closest to an eyeball, effects of volatile medicines are utilized to the maximum extent, and a novel approach is developed for eye medication. The volatile medicines are anti-inflammatory, applicable to anti-pathogenic microorganisms, and capable of eliminating asthenopia, relieving dry symptoms of eyes and eliminating xerophthalmia. An opening is arranged on only one side of a rotary wheel (a through hole is arranged on only one side of a thin film). When the adjustable volatile cell is not used, the twisting wheel is twisted to drive the rotary wheel so as to rotate the opening side into the groove, and accordingly dust is prevented from falling on a carrier for carrying medicine liquids, and the medicine liquids can be prevented from continuing to volatize.
Owner:崔浩 +1
Volatile medicine dosing device with eye health caring effect
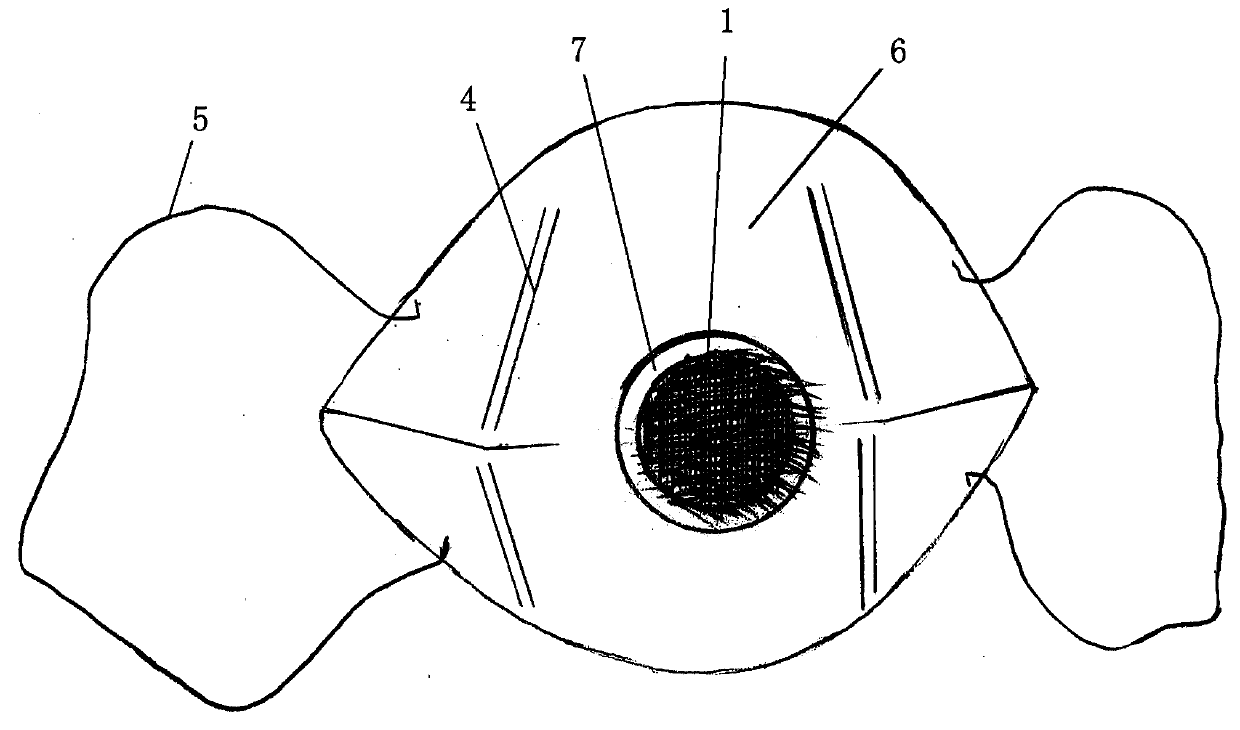
InactiveCN105534638APlay a health functionConducive to fullEye treatmentVolatile drugsBiomedical engineering
The invention provides a volatile medicine dosing device with the eye health caring effect. The medicine dosing device is a head-wearing-type device. The head-wearing-type device is worn to the head and comprises a body structure and a fixing device. The body structure comprises a shell surrounding the two eyes at the same time; the fixing device is wound around the back of the head or is hung to the double ears; volatile medicine storing devices are arranged at the portions, corresponding to the peripheries of the eyes of the human body, of the interior of the shell. According to the medicine dosing device, traditional dosing modes such as an eye hot-compressing mode or an eye liquid dropping mode are broken through, the head-wearing-type device is adopted, volatile medicine is stored to the portions, corresponding to the peripheries of the eyes of the human body, of the interior of the shell of the head-wearing-type device, the medicine dosing device can be used for various myopia therapeutic apparatuses and particularly can be used for various amblyopia therapeutic apparatuses, the effect of the medicine is developed while the eyes are trained,, and convenience and practicality are achieved.
Owner:HANGZHOU LUKE TECH CO LTD
Wearable portable atomization mask
PendingCN112450520AExtended service lifeSkin-friendly adhesiveMedical atomisersInhalatorsMedication injectionPharmacy medicine
The invention discloses a wearable portable atomization mask which comprises a mask body, a volatile medicine breathing channel is formed in the mask body, the volatile medicine breathing channel penetrates through the mask body, a liquid medicine injection storage tube is arranged on one side of the volatile medicine breathing channel, and a flexible solar cell panel is further arranged on the mask body; a control button is arranged at the bottom of the flexible solar cell panel, and a two-dimensional code used for recognizing medicine information is arranged on one side of the control button. When the mask is used, medicine injection, atomization and disinfection are integrated, the complexity of the device is reduced, and the operation is easy and convenient; accurate administration isperformed according to related data, so that the drug effect is improved; an atomization mode and a mask mode are adopted, and one mask has multiple functions; the periphery of the mask is attached toskin, a lacing structure of a traditional mask is omitted, and the requirements of special crowds are met; and the mask is simple and compact in structure, light and practical.
Owner:XIAN MEDICAL UNIV
Warm moxibustion plaster
PendingCN110693707AEvenly heatedGood treatment effectDevices for heating/cooling reflex pointsMedical devicesGraphene flakeEngineering
The invention relates to a plaster, in particular to a warm moxibustion plaster. The invention aims to solve the problem that all medicine molecules in an ointment layer are fully heated difficultly by an existing warm moxibustion plaster. Therefore, the warm moxibustion plaster comprises a base layer, a graphene electric heating ointment layer and a heat conduction insulating layer which are sequentially laminated from top to bottom; the graphene electric heating ointment layer comprises a graphene conductive network and volatile medicine particles attached to the graphene conductive network;the graphene conductive network is formed by paving a graphene conductive agent; the graphene conductive agent comprises a plurality of graphene sheets, and the graphene sheets can be attached to theoutsides of the volatile drug particles to attach the volatile drug particles to the graphene conductive network, namely, the medicine particles are dispersed throughout the whole graphene conductivenetwork; and when the conductive network forms infrared radiation, each volatile medicine particle attached on the conductive network can be rapidly and uniformly warmed to an appropriate temperature, and therefore, the treatment effect of the warm moxibustion plaster is greatly improved.
Owner:BEIJING TAIYI TECH CO LTD
Gas phase extraction and enrichment method for liquid phase volatile material, and system thereof
ActiveCN102847339AHigh enrichment efficiencyEfficient enrichmentLiquid degasificationSolvent extractionEnvironment waterEnrichment methods
The present invention relates to a gas phase extraction and enrichment method for a liquid phase volatile material, and a system thereof. The system comprises a liquid-gas extraction unit and a gas phase compression enrichment unit, wherein the liquid-gas extraction unit adopts a specific gas as a working gas to extract a target volatile substance to a gas phase from the liquid phase so as to achieve liquid-gas phase transfer of the target volatile substance, and the gas phase compression enrichment unit adopts a special working liquid to absorb the working gas, such that a volume of the working gas containing the extracted target volatile substance is significantly reduced so as to achieve the gas phase compression enrichment of the target volatile substance. The method and the system in the present invention have good application prospects in volatile drug extraction, environment water body volatile pollutant enrichment and monitoring, and other fields.
Owner:FUZHOU UNIV
Slow releasing device for volatile medicine and air conditioner using same
There is provided a volatile medicine controlled release member capable of equalizing and controlling volatile release of medicine at a low concentration release speed, preventing unnecessary release by sufficiently dealing with a humidity change, and having a structure wherein a volatile volume is hardly affected even when a liquid volume of the medicine falls. The volatile medicine controlled release member is provided with a container with an interior filled with liquid medicine, a liquid absorber comprising a medium for sucking up the medicine, a space part for filling an interior with the medicine volatilized from the liquid absorber, and a humidity sensitive film with a permeation amount of the medicine changing in response to the humidity change. The medicine is sucked up and volatilized by the liquid absorber, and it is controllably released from the humidity sensitive film in response to the humidity change while it is retained at certain concentration in the space part formed by the liquid absorber and the humidity sensitive film.
Owner:PANASONIC CORP
Migraine treating instrument
The invention discloses a migraine treating instrument. The instrument comprises a head band and a fixing seat, the fixing seat clamps the two ends of the head band, a vibrator is embedded in the surface of one side of the fixing seat, the surface of one side of the vibrator mutually adheres to the inner wall of the head band, an application treatment box is bonded to the surface of the inner wallof the head band, seepage holes are formed in the surface of the application treatment box, absorbing cottons are bonded to the surface of the application treatment box, a feeding port is formed in the surface of the top of the application treatment box, and the surface of the head band is wrapped with a protecting rubber ring. By arranging the application treatment box, volatile medicines whichcan relieve migraine are conveniently added to the application treatment box through the feeding port formed in the surface of the top of the application treatment box, hot water or cold water can beadded, liquid seeps out to the absorbing cottons through the seepage holes formed in the surface of the application treatment box to perform application treatment on a patient, and therefore the painof the patient can be effectively relieved.
Owner:NANJING ZHENGLIANG MEDICAL TECH
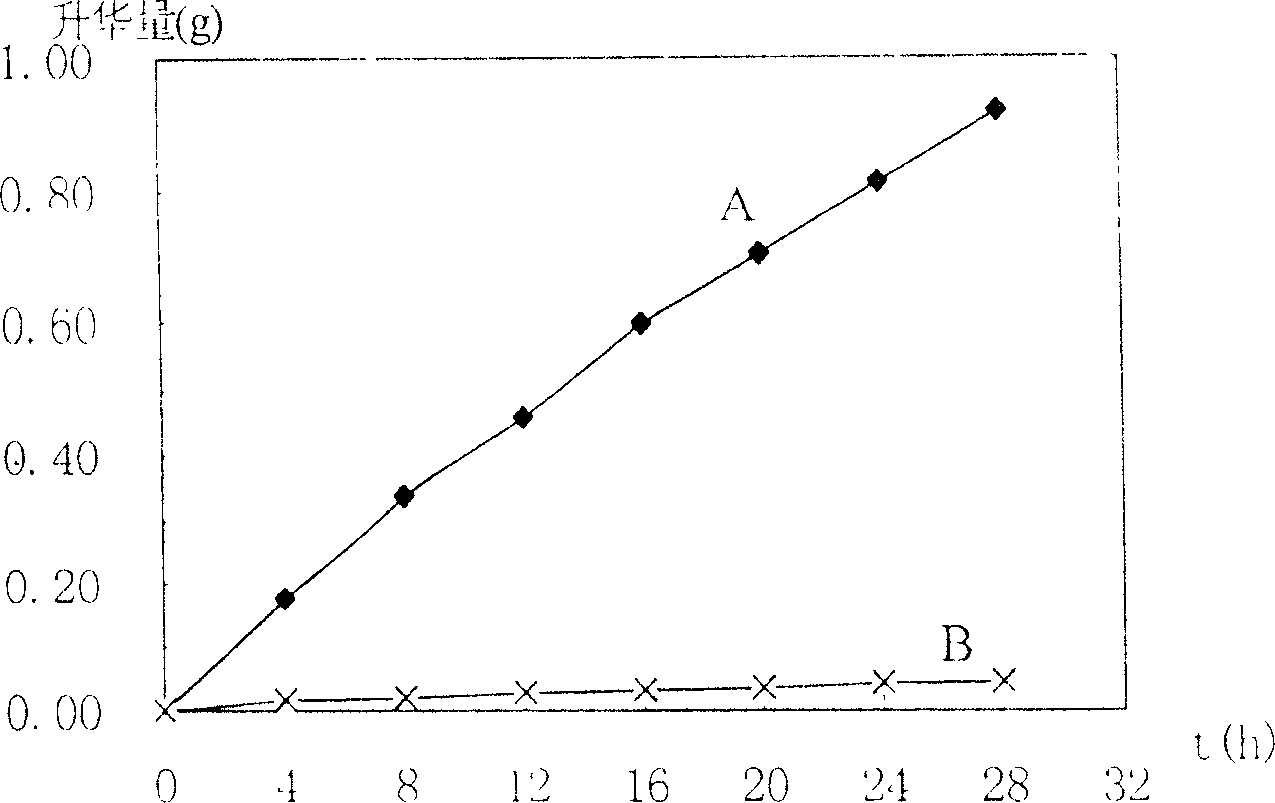
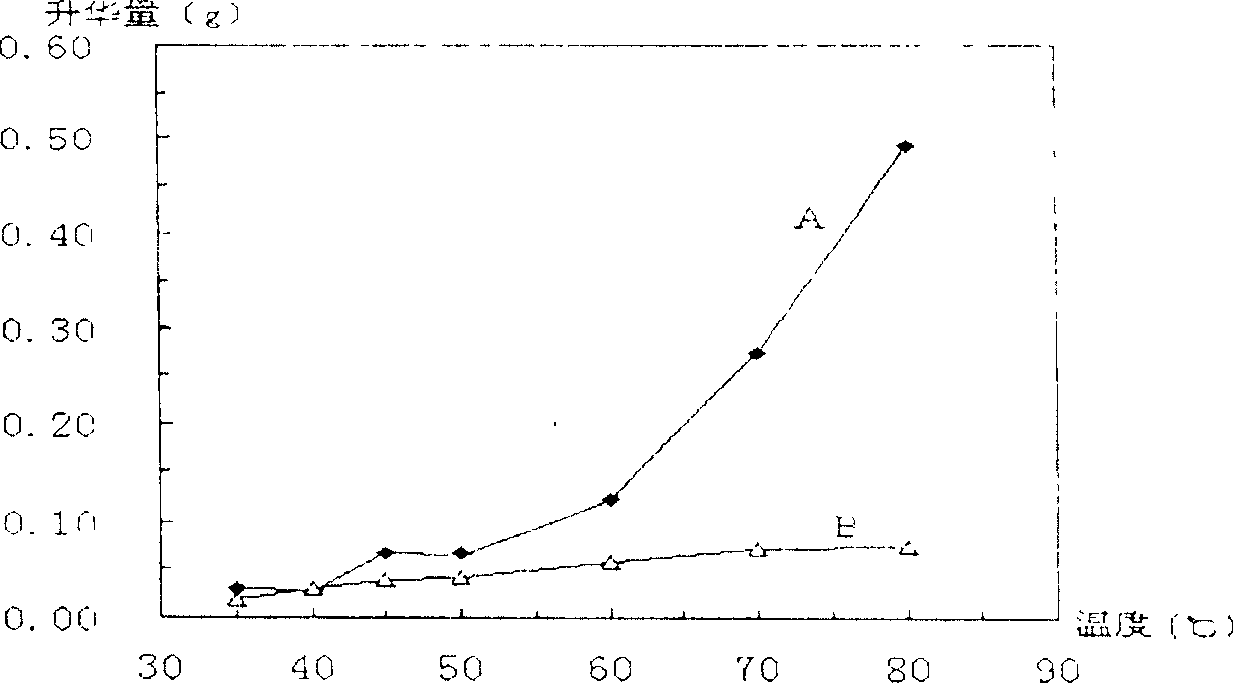
Preparation method of emplastrum matrix capable of rapidly releasing volatile drugs
ActiveCN114796164AQuick releaseEasy to prepareHydroxy compound active ingredientsAntipyreticElastomerWater methanol
The invention relates to a preparation method of an emplastrum matrix capable of rapidly releasing volatile drugs, the emplastrum matrix is prepared by matching an SIS framework material with liquid paraffin, an antioxidant BHT and hydrogenated rosin glyceride, and the preparation method comprises the following steps: (1) synthesizing a linear SIS block copolymer; b, after the first block polymerization is completed, initiating a second block polymerization reaction; c, after the second block polymerization is completed, initiating a third block polymerization reaction; d, after the third block polymerization reaction is finished, 900-1100 ml of absolute methanol is added to terminate the reaction to obtain an SIS elastomer, the SIS elastomer is placed in a vacuum oven and dried at 48-52 DEG C for 10-14 h, and the dried SIS elastomer is obtained; and (2) preparing an emplastrum matrix. The preparation method disclosed by the invention is simple, can realize quick release of the medicine, has an accumulated release rate obviously higher than that of a process sample of an original prescription, not only reduces resource waste, but also provides a feasible method for improving the potential curative effect of a product, and has remarkable social and economic benefits.
Owner:HENAN LINGRUI PHARMA
Gas phase extraction and enrichment method for liquid phase volatile material, and system thereof
ActiveCN102847339BHigh enrichment efficiencyEfficient enrichmentLiquid degasificationSolvent extractionEnvironment waterEnrichment methods
The present invention relates to a gas phase extraction and enrichment method for a liquid phase volatile material, and a system thereof. The system comprises a liquid-gas extraction unit and a gas phase compression enrichment unit, wherein the liquid-gas extraction unit adopts a specific gas as a working gas to extract a target volatile substance to a gas phase from the liquid phase so as to achieve liquid-gas phase transfer of the target volatile substance, and the gas phase compression enrichment unit adopts a special working liquid to absorb the working gas, such that a volume of the working gas containing the extracted target volatile substance is significantly reduced so as to achieve the gas phase compression enrichment of the target volatile substance. The method and the system in the present invention have good application prospects in volatile drug extraction, environment water body volatile pollutant enrichment and monitoring, and other fields.
Owner:FUZHOU UNIV
A high-efficiency continuous medicine drying device
ActiveCN113405326BDrying helpsKeep dryDrying solid materials without heatDrying machines with local agitationInsulation layerDesiccant
The invention relates to a high-efficiency and continuous medicine drying device, which comprises a drying device body, the drying device body includes a device cavity and a device shell arranged on the surface of the device cavity, a discharge port is arranged at the upper port of the device cavity, and the device The lower port of the cavity is provided with a feed port, the inside of the device cavity is provided with a deflector on the side close to the discharge port, and the side of the device cavity close to the feed port is provided with a diffuser, a deflector and A desiccant is filled between the diffuser plates. The present invention prepares a kind of medicine drying equipment, which can have a good drying effect on gas or volatile medicines, and overcomes the relatively high risk, low dehydration efficiency and poor dehydration effect existing in the drying of gas medicines. Defects. The diffuser plate, deflector plate and desiccant provided in the present invention all contribute to the drying of the gas, and the purpose of the heating layer and the thermal insulation layer is to provide a suitable drying environment for the drying device.
Owner:北京市永康药业有限公司
Multifunctional flexible cultural porcelain pillow
InactiveCN101219027AVentilated and coolWith deflection degrees of freedomPillowsHuman bodyMetallurgy
The invention relates to a multifunctional soft ceramic pillow which consists of a pillow core and a pillowslip, wherein, a plurality of hollow rivets are embedded on the pillowslip and a ceramic button is embedded on the central hole of each hollow rivet; the button holes of a plurality of ceramic buttons are integrated into a whole through series connection cores. The pillowslip made by the connection of a plurality of ceramic buttons is cool and easy to clean due to that the pillowslip never absorb sweat from human body; hollow ceramic chips provided with volatilization holes are embedded on a plurality of hollow rivets at the periphery of the pillowslip; perfume, essential balm or volatile drugs are filled in the cavity of the hollow ceramic chips to refresh the air and improve the slumber of people; images and characters fired on the ceramic buttons or the images or characters formed by a plurality of ceramic buttons has strong impression and rich cultural connotations, therefore, the ceramic pillow not only is a practical decoration with grade but also a cultural carrier.
Owner:韩国斌
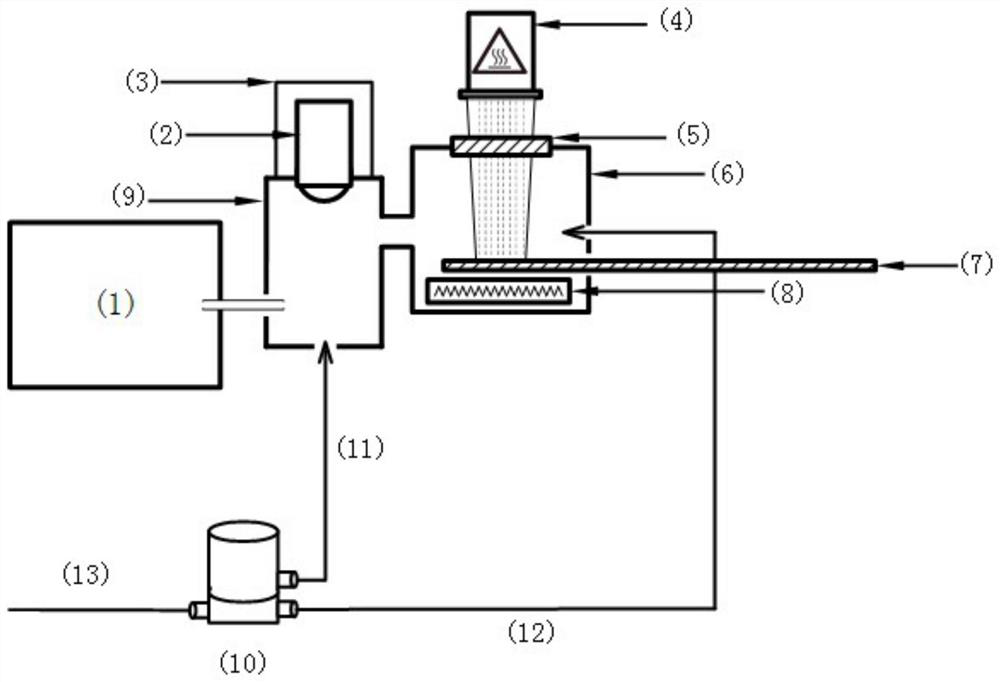
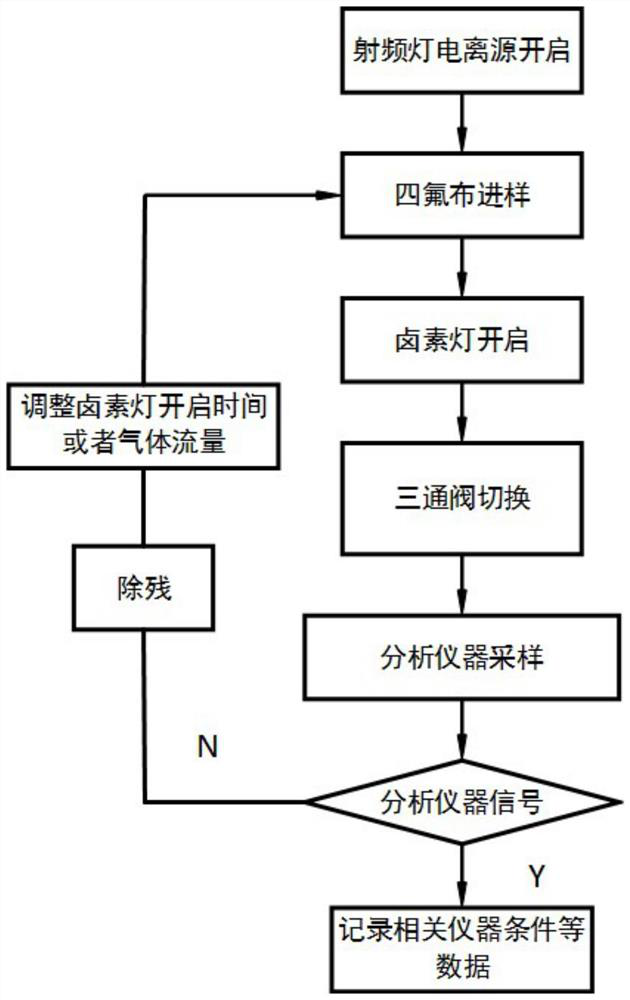
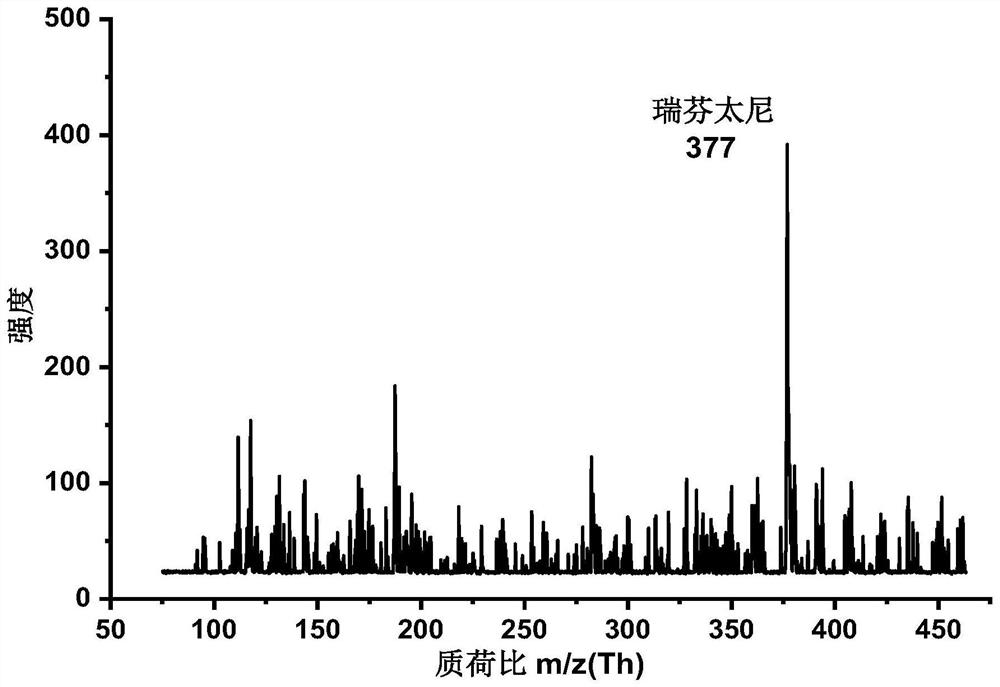
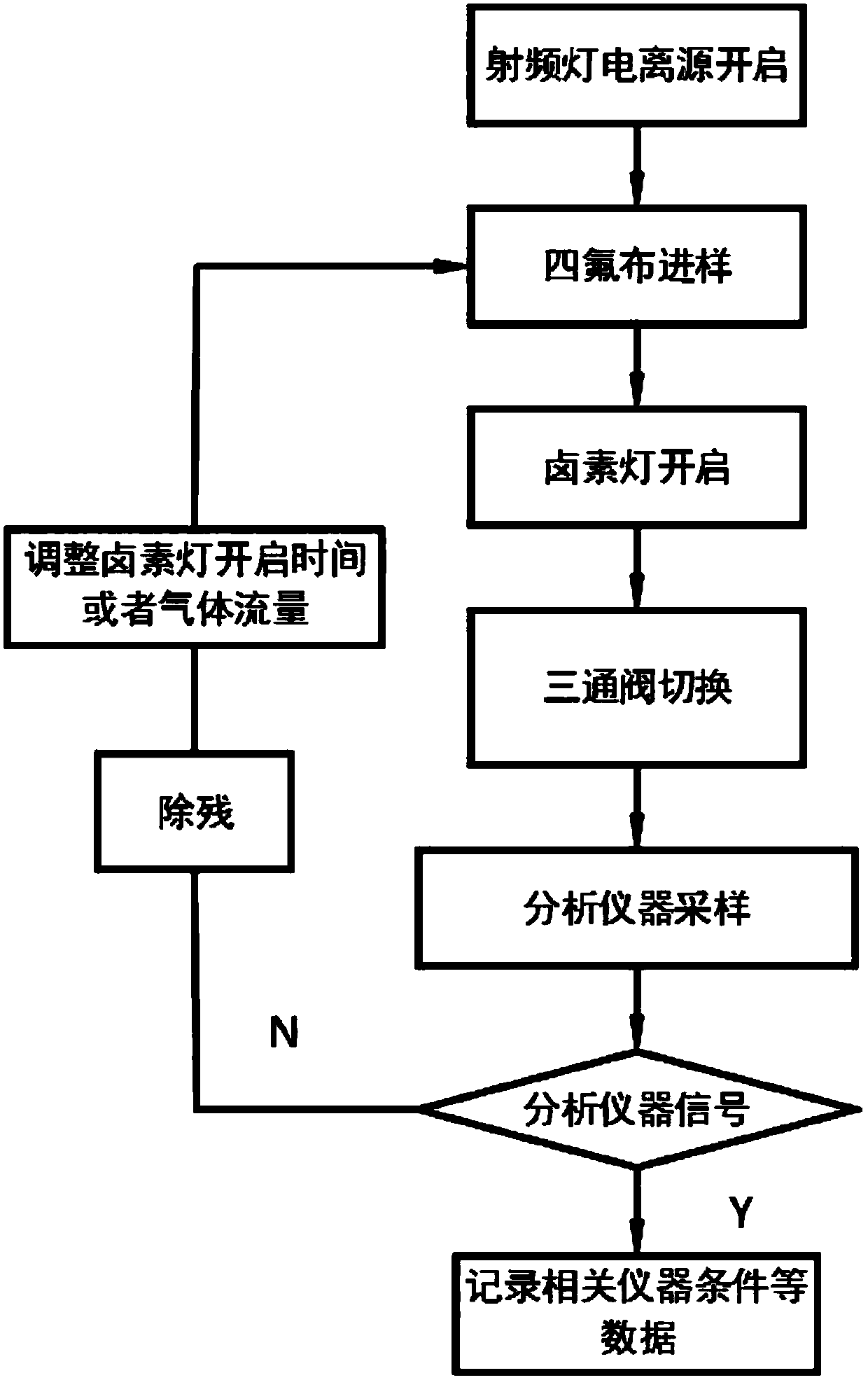
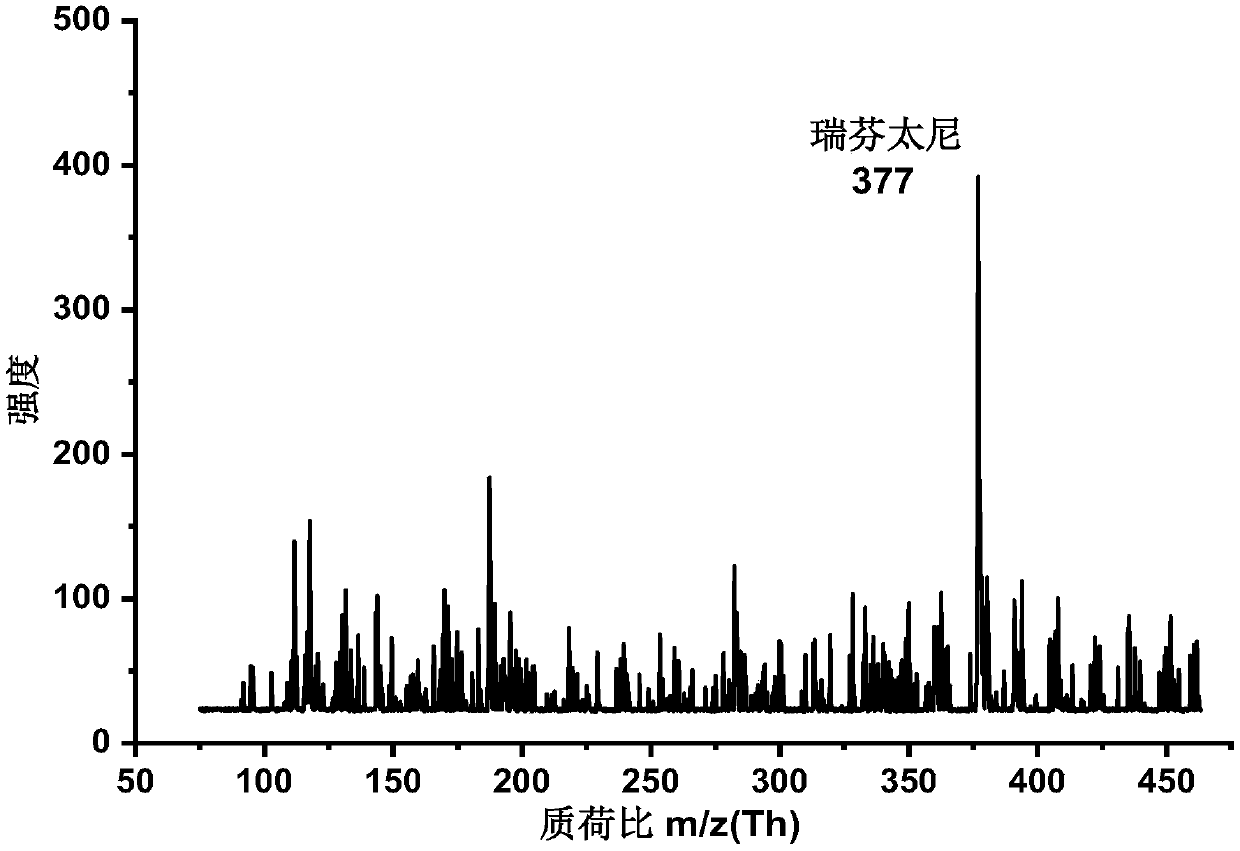
A photoflash thermal analysis-delayed purge injection method for the detection of drug mixtures
ActiveCN111105980BIncrease concentrationFast heatingSamples introduction/extractionMaterial analysis by electric/magnetic meansPsychoactive drugOpiate
The invention discloses a light flash thermal analysis-delay purging sample introduction method for rapid and simultaneous detection of volatile and low-volatile drug mixtures. There are many types of drugs with large differences in structure and molecular weight, and some of them are difficult to volatile drugs. They will reduce the sensitivity of drug detection instruments that use thermal analysis to inject samples, and at the same time affect the detection and identification efficiency of opium or new psychoactive drug mixtures. The present invention solves the problem of rapid and simultaneous detection of volatile-refractory drug mixtures. The analysis method uses a halogen lamp to perform "flash heat" to rapidly heat up the drug mixture and increase the vapor pressure of the mixed sample, thereby increasing the atmospheric pressure. The ionization efficiency increases the signal intensity of less volatile samples in the mixture by more than an order of magnitude. Combined with the time-delay purging device, the difficult-to-volatile and volatile substances volatilized during the flash heat are purged into the ionization chamber at the same time, which reduces the loss of samples during the continuous purging and heating process, and realizes the difficult-to-volatile and volatile substances. Simultaneous detection of both.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Drug detector based on animal olfactory sensation
The disclosed drug detector based on animal olfaction comprises: a CCD camera, a brain stereotaxic instrument, an electrode, a pre-amplification and filter circuit, a multiarm maze, and computer control and analysis system. Wherein, according to animal brain olfaction cortex neuron electric action, letting animal produce specific memory to volatile drug; random putting drug in small rooms of the maze to give prize stimulation for specific brain area and reinforce the memory till animal action includes statistic meaning, collecting relative induced brain electric information to input computer for recognition and analysis and form training database.
Owner:XI AN JIAOTONG UNIV
A kind of veterinary pefloxacin mesylate pellets and preparation method thereof
InactiveCN102258478AImprove bioavailabilityMask bitternessAntibacterial agentsOrganic active ingredientsIrritationVolatile drugs
The invention relates to animal pefloxacin mesylate pellets, and a preparation method thereof. The preparation method of the animal pefloxacin mesylate pellets comprises steps that: pefloxacin mesylate powder, a wall material which is a high-molecular material, and cyclohexane are placed in an organic solvent reaction vessel, and reflux is carried out for 1 to 2 hours under a temperature of 80 to85 DEG C; the temperature of the materials is reduced to 25 to 35 DEG C at a speed of 1 to 3 DEG C per 5 minutes, such that pefloxacin mesylate is coated with the wall, and pellets with sizes of 40 to 200 meshes are formed; the pellets are vacuum-dried, such that the animal pefloxacin mesylate pellets are obtained. According to the animal pefloxacin mesylate pellets provided by the invention, a high-molecular material or polymer is coated on the surface of the medicine, such that tiny sealed capsules are obtained, and the capsules serve as covers or protective films. Therefore, the bitter taste of the medicine can be covered; medicine stability can be improved; and volatilization of volatile medicines can be controlled. The animal pefloxacin mesylate pellets provided by the invention has advantages of convenient use, simple production technology, low cost, high medicine bioavailability, low irritation to intestines and stomach, and good medicine stability.
Owner:WUHAN HUAYANG ANIMAL PHARMA
Chinese medicine decocting preparation technique for preventing volatile ingredients from losing
InactiveCN101284023BImprove utilizationIncrease concentrationPlant ingredientsMedicineAdditive ingredient
The invention relates to a method for preparing Chinese medicinal decoction capable of improving the availability of Chinese medicinal raw materials and preventing the loss of volatile components. The method comprises the steps of: adding Chinese medicinal materials into a sealed reaction kettle, heating, allowing volatile components to be condensed in the condenser tube of a condenser and flow back to the reaction kettle, rapidly cooling the reaction kettle, adding cosolvent, and subpackaging in sealed containers. The method can keep insoluble and volatile effective components in the decoction, so as to increase the concentration of effective components; and can maximally recover heat energy produced in decoction process, so as to reduce energy consumption. The obtained decoction has improved quality and therapeutic effect.
Owner:GUANGXI DOUBLE ANT PHARMA
Light flash thermal desorption-delayed purging sample injection method for drug mixture detection
ActiveCN111105980AIncrease concentrationFast heatingSamples introduction/extractionMaterial analysis by electric/magnetic meansOpiatePhysical chemistry
The invention discloses a light flash thermal desorption-delayed purging sample injection method for rapidly and simultaneously detecting volatile and non-volatile drug mixtures. A plurality of typesof drugs exist, and are large in difference of the structures and the molecular weights; and some drugs, which are not easy to volatilize, can reduce the sensitivity of a drug detection instrument utilizing thermal desorption sample injection, and meanwhile, influence the detection and identification efficiency of opium or new psychologically active drug mixtures. According to the method, the problem of rapid and simultaneous detection of the volatile-non-volatile drug mixture is solved; a halogen lamp is used for flash heating, so that the drug mixture is rapidly heated, the vapor pressure ofthe mixed sample is increased, the ionization efficiency at normal pressure is improved, and the signal intensity of the non-volatile sample in the mixture is improved by one order of magnitude or more; and by combining with a delayed purging device, the non-volatile and volatile substances volatilized during flash heating are purged into an ionization cavity at the same time, so that the loss ofsamples in the continuous purging and heating process is reduced, and the simultaneous detection of non-volatile and volatile drugs is realized.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
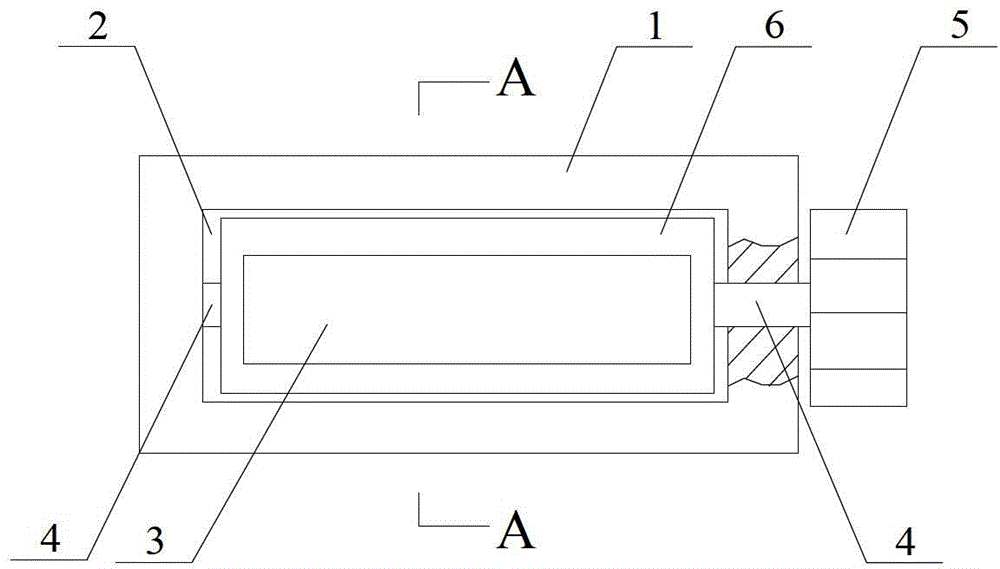
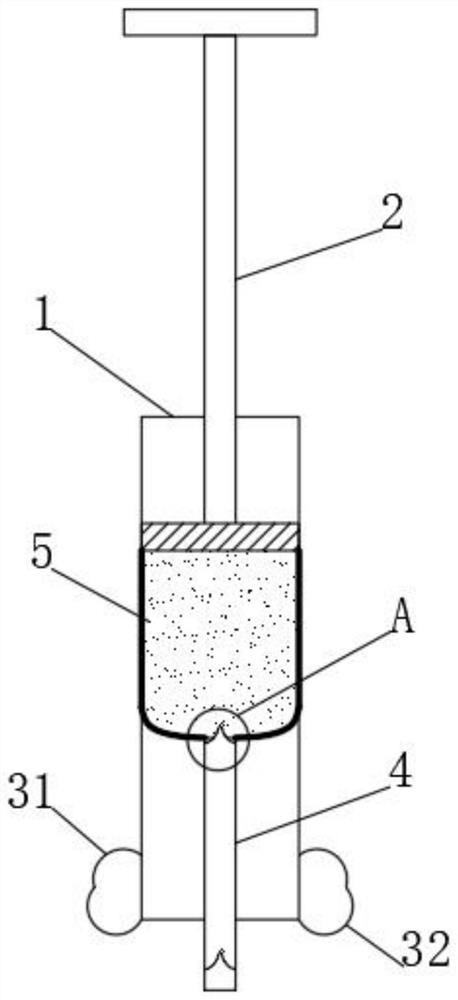
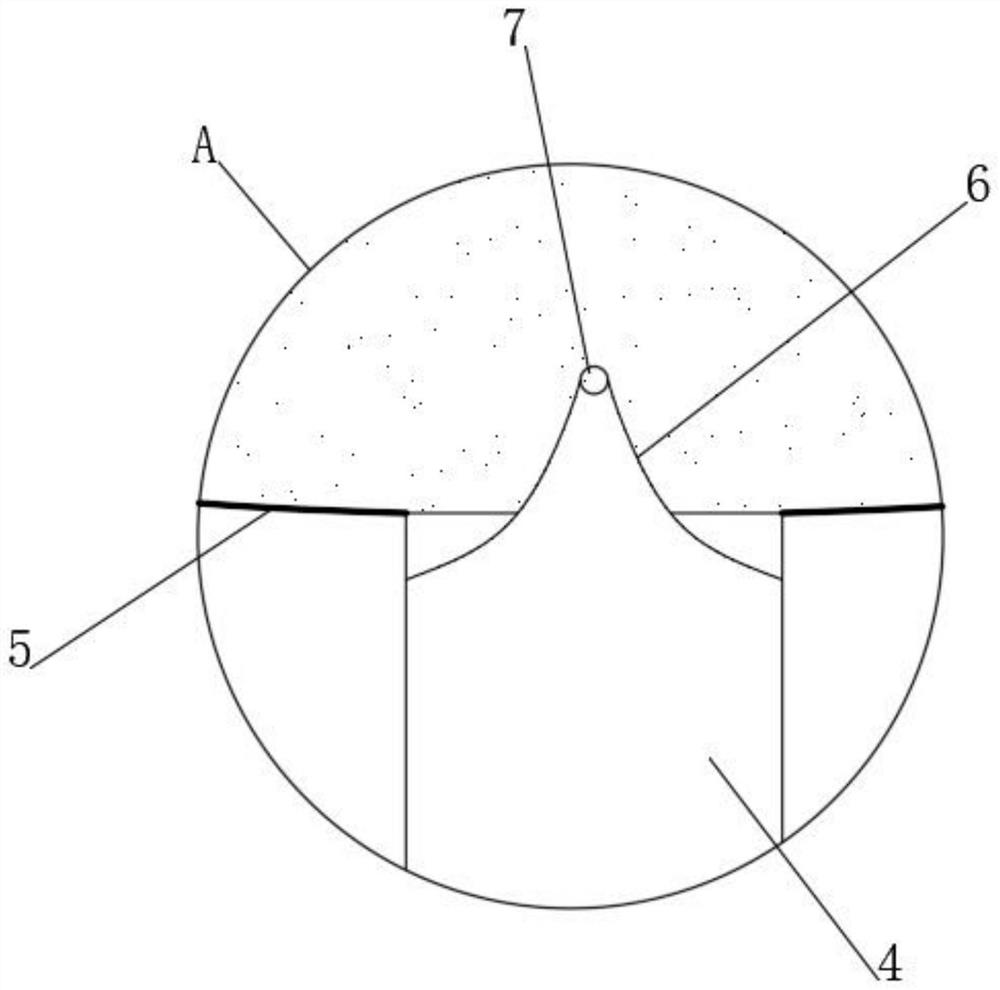
Self-spill-proof extractor for volatile medicines
PendingCN112791243APrevent volatilizationNot easy to oxidizeSuction devicesMedicineBiochemical engineering
The invention discloses a self-spill-proof extractor for volatile drugs, and belongs to the field of extractors. The self-spill-proof extractor for the volatile drugs is characterized in that through the arrangement of a bidirectional containing bag and a turning notch piece, when the drugs are extracted, a drug suction pipe is firstly inserted into an opening part of a container for containing the drugs, then inert gas is extruded into a container bottle, on one hand, the empty space in the container after the medicine is sucked can be effectively filled, then volatilization of the medicine is effectively restrained, on the other hand, the inert gas can effectively protect unstable volatile gas from being prone to oxidation reaction with air, when the medicine is sucked, the turning notch piece reversely deforms, the medicine enters the two-way containing bag, in addition, under the action of an outer isolation bag, the outside and the extraction opening in the container opening can be effectively isolated, and then medicine is effectively prevented from being volatilized to the outside during medicine extraction.
Owner:LIAONING INST OF SCI & TECH
Air conditioner
There is provided a volatile medicine controlled release member capable of equalizing and controlling volatile release of medicine at a low concentration release speed, preventing unnecessary release by sufficiently dealing with a humidity change, and having a structure wherein a volatile volume is hardly affected even when a liquid volume of the medicine falls. The volatile medicine controlled release member is provided with a container with an interior filled with liquid medicine, a liquid absorber comprising a medium for sucking up the medicine, a space part for filling an interior with the medicine volatilized from the liquid absorber, and a humidity sensitive film with a permeation amount of the medicine changing in response to the humidity change. The medicine is sucked up and volatilized by the liquid absorber, and it is controllably released from the humidity sensitive film in response to the humidity change while it is retained at certain concentration in the space part formed by the liquid absorber and the humidity sensitive film.
Owner:PANASONIC CORP
High-activity slow-release pain-relieving patch and preparation process thereof
PendingCN113476459AHigh activityGuaranteed validityHydroxy compound active ingredientsAntipyreticTransdermal patchMethyl salicylate + menthol
The invention relates to a high-activity slow-release pain-relieving patch and a preparation process thereof. The high-activity slow-release pain-relieving patch comprises a backing layer, a main body layer and an isolation layer which are sequentially stacked, wherein the main body layer comprises the following components: a matrix, active ingredients and an active ingredient slow-release inclusion material, wherein the matrix comprises 30-50wt% of a framework material and 12-28wt% of an auxiliary agent; the active ingredients comprise 6-12wt% of methyl salicylate, 5-8wt% of menthol and 1-4wt% of camphor; and the active ingredient slow-release inclusion material accounts for 6-18wt%. According to the high-activity slow-release pain-relieving patch, medicines with high activity and high volatility, namely methyl salicylate, menthol and camphor, are selected as the main body of the high-activity slow-release pain-relieving patch, and the active ingredients in the main body are included by adopting the slow-release inclusion material, so that the content of each active ingredient is kept to the maximum extent and is continuously and stably released, and the effectiveness of the medicines is ensured; and the matrix material with low sensitization and hydrophilicity is selected, and the preparation process adopts an innovative solvent-free production process technology, so that the transdermal patch is not easily allergic to a human body when being used for the human body, and the safety of the medicines is ensured.
Owner:ZHEJIANG DINGTAI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com