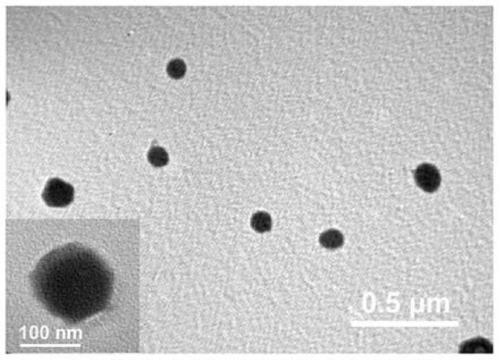
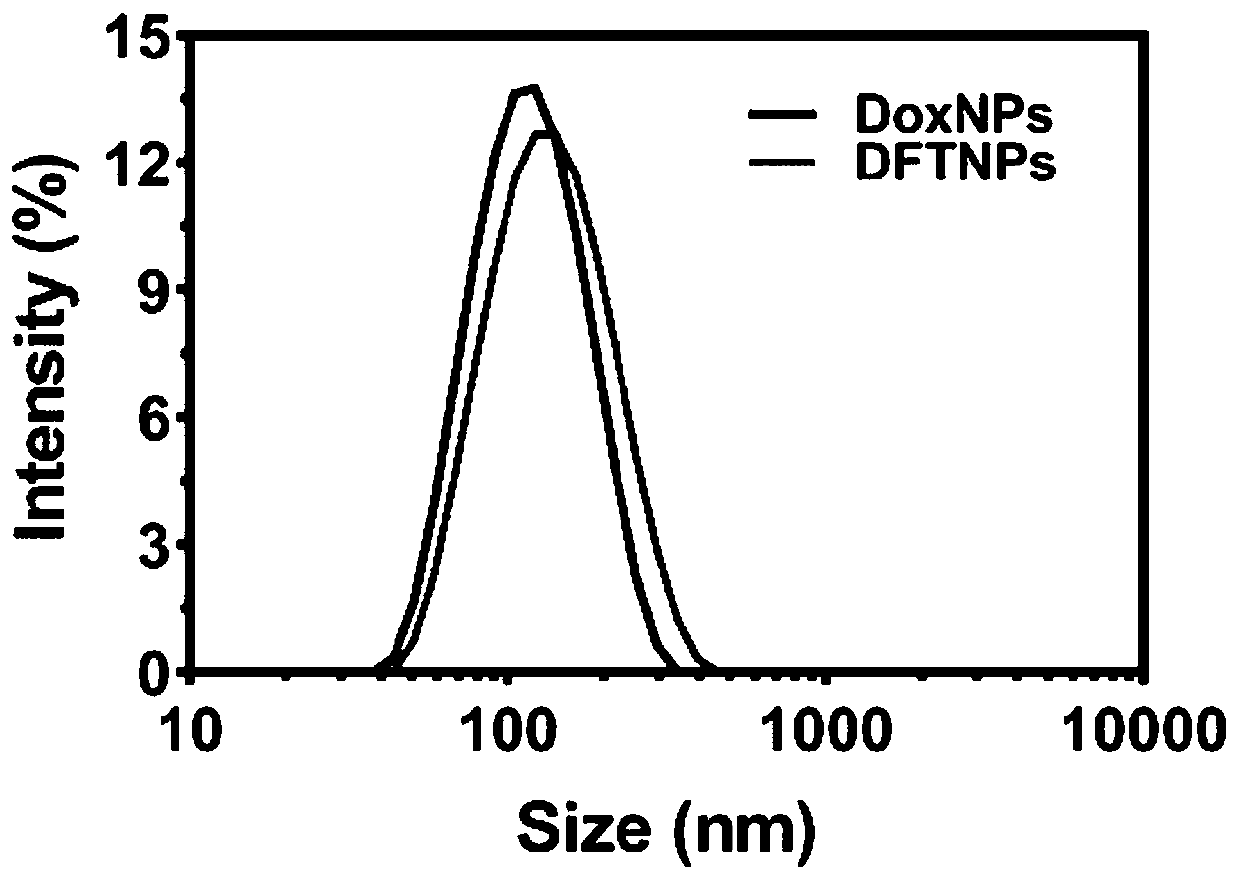
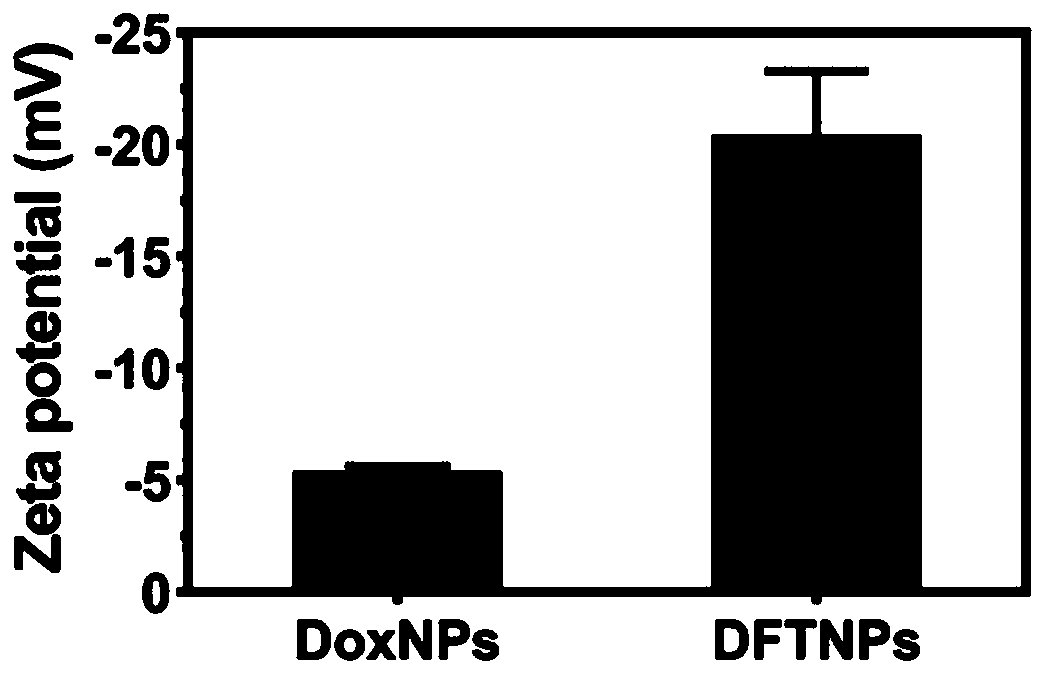
Application of nanoparticles as tumor microenvironment responsive drug or imaging agent
A tumor microenvironment and nanoparticle technology, which is applied in the application field of nanoparticles as tumor microenvironment responsive drugs or imaging agents, can solve the problem of inability to realize real-time monitoring of chemotherapy drugs, inability to realize fixed-point release of chemotherapy drugs, and not to mention chemotherapy drugs. Control of release rate and other issues to prevent premature inactivation, increase cycle time, and reduce toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the preparation of nanoparticle
[0063] 10 mg of DOX·HCl (dobiraxin hydrochloride) was dissolved in 10 ml of DMSO (dimethyl sulfoxide) solution. Then stir moderately at room temperature, add 1ml of trimethylamine, remove hydrochloric acid to convert hydrophilic DOX·HCl into hydrophobic DOX nanoparticles, and obtain a mixture after uniform stirring. Then 100 μl of the prepared mixture (DOX / DMSO solution neutralized by trimethylamine) was dripped into 980 μl of high-purity water under vigorous stirring (stirring at a speed of 700 rpm), and the dropping rate of high-purity water was 800 μl / min to obtain a dispersion. Then 10 μl of Ta (tannic acid) solution (40 mg / ml) and 10 μl of FeCl 3 ·6H 2 O solution (20 mg / ml) was sequentially added to the above dispersion, then sonicated for 50 s, and neutralized with 1 μM NaOH solution. The resulting product was centrifuged with deionized water to remove excess TA and FeCl 3 . The nanoparticles were redispersed in...
Embodiment 2
[0066] 10 mg of DOX·HCl (dobiraxin hydrochloride) was dissolved in 10 ml of DMSO (dimethyl sulfoxide) solution. After moderate stirring at room temperature, 1 ml of trimethylamine was added, and hydrochloric acid was removed to convert hydrophilic DOX·HCl into hydrophobic DOX nanoparticles. Then, 100 μl of the prepared DOX / DMSO solution was dripped into 980 μl of high-purity water under vigorous stirring (stirring at a speed of 1000 rpm), and the dropping speed of the high-purity water was 1000 μl / min to obtain a dispersion. Then 100 μl of Ta (tannic acid) solution (40 mg / ml) and 100 μl of FeCl 3 ·6H 2 O solution (20 mg / ml) was sequentially added to the above dispersion, then sonicated for 50 s, and neutralized with 1 μM NaOH solution. The resulting product was centrifuged with deionized water to remove excess TA and FeCl 3 . The nanoparticles were redispersed in high-purity water to obtain DFTNPs.
Embodiment 3
[0068] 10 mg of DOX·HCl (dobiraxin hydrochloride) was dissolved in 10 ml of DMSO (dimethyl sulfoxide) solution. After moderate stirring at room temperature, 1 ml of trimethylamine was added, and hydrochloric acid was removed to convert hydrophilic DOX·HCl into hydrophobic DOX nanoparticles. Then, 100 μl of the prepared DOX / DMSO solution was dripped into 980 μl of high-purity water under vigorous stirring (stirring at a speed of 300 rpm), and the dropping rate of the high-purity water was 100 μl / min to obtain a dispersion. Then 50 μl of Ta (tannic acid) solution (40 mg / ml) and 50 μl of FeCl 3 ·6H 2 O solution (20 mg / ml) was sequentially added to the above dispersion, then sonicated for 50 s, and neutralized with 1 μM NaOH solution. The resulting product was centrifuged with deionized water to remove excess TA and FeCl 3 . The nanoparticles were redispersed in high-purity water to obtain DFTNPs.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com