Light-controlled temperature-sensitive liposome and its preparation method and application
A temperature-sensitive lipid and liposome technology, applied in the field of medicine and chemical industry, can solve the problems of difficult and rapid release of drugs, and the inability to accurately control physiological temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of PEGylated Photosensitive Molecule 2
[0061] Take 57 mg of photosensitive molecules shown in formula 2, 26.9 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 19.0 mg of 1-hydroxybenzotriazole and add them to 20 mL of tetrahydrofuran and In a mixed solvent of 1mL N,N-dimethylformamide, seal and react at room temperature for 2h. Then, dissolve 36.7mg of GFLG short peptide (glycine-phenylalanine-leucine-glycine) in 2mL tetrahydrofuran to make a mixed solution, then add this mixed solution to the above reaction solution, and continue the closed reaction at room temperature 24h. The organic solvent was removed by rotary evaporation, and the obtained substance was dissolved in methanol, separated and purified by preparative liquid chromatography, precipitated and crystallized with ether, and the photosensitive molecule 2 modified by GFLG was obtained. Add 50mg of GFLG-modified photosensitive molecule 2, 11.6mg of 1-(3-dimethyla...
Embodiment 2
[0062] Example 2: Preparation of PEGylated Photosensitive Molecule 3
[0063] Take 68 mg of the photosensitive molecule shown in formula 3, 26.9 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 19.0 mg of 1-hydroxybenzotriazole and add them to 20 mL of tetrahydrofuran and In a mixed solvent of 1mL N,N-dimethylformamide, seal and react at room temperature for 2h. Then 54.6mg proline-leucine-glycine-leucine-alanine-glycine (PLGLAG) was dissolved in 2mL tetrahydrofuran to make a mixed solution, and then this mixed solution was added to the above reaction solution, and continued to Airtight reaction at room temperature for 24h. The organic solvent was removed by rotary evaporation, the resulting substance was dissolved in methanol, separated and purified by preparative liquid chromatography, and ether precipitated and crystallized to obtain PLGLAG-based photosensitive molecule 3, and then 67.7 mg PLGLAG-based photosensitive molecule 2, 11.6 mg 1-(3- Dimethyl...
Embodiment 3
[0064] Example 3: Preparation of PEGylated Photosensitive Molecule 4
[0065]Take 50 mg of the photosensitive molecule shown in formula 4, 26.9 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 19.0 mg of 1-hydroxybenzotriazole and add it to 20 mL of tetrahydrofuran and In a mixed solvent of 1mL N,N-dimethylformamide, seal and react at room temperature for 2h. Then, dissolve 421 mg of aminated polyethylene glycol monomethyl ether and 12 μL of anhydrous triethylamine in 10 mL of dichloromethane to make a mixed solution, then add the mixed solution to the above reaction solution, and continue the sealed reaction at room temperature 24h. The organic solvent was removed by rotary evaporation, and the obtained substance was dissolved in methanol, separated and purified by preparative liquid chromatography, precipitated and crystallized with ether, and PEGylated photosensitive molecule 4 was obtained.
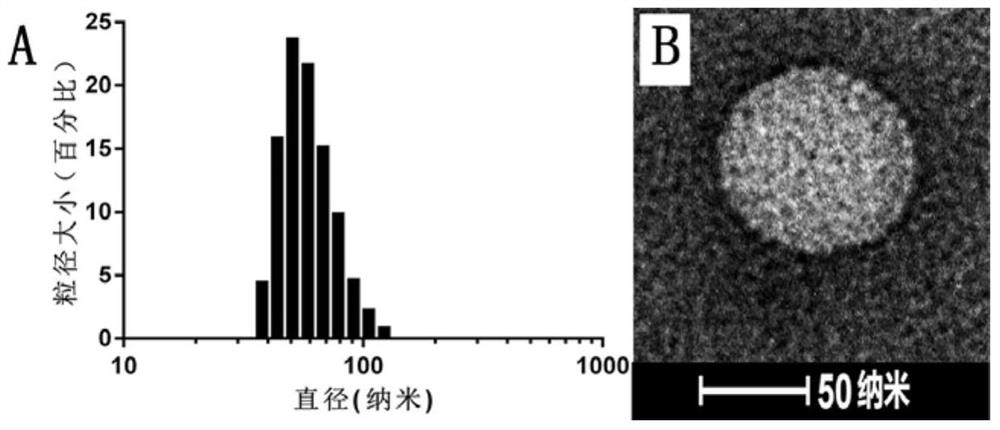
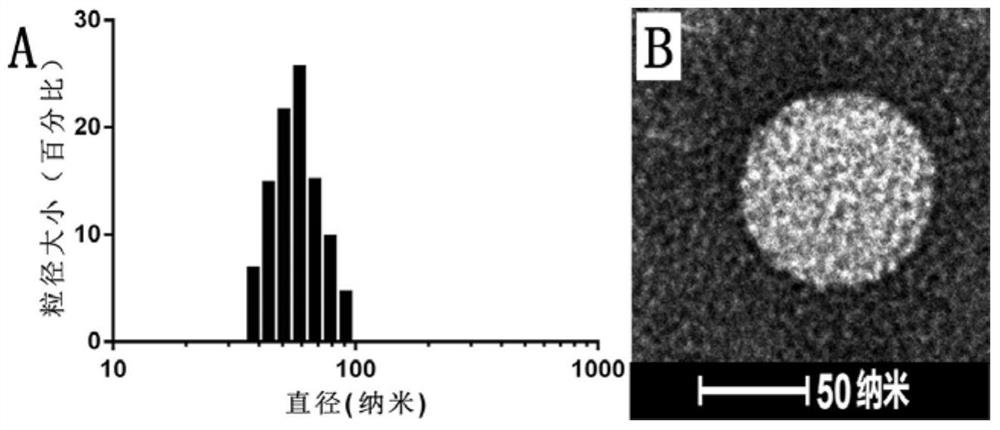
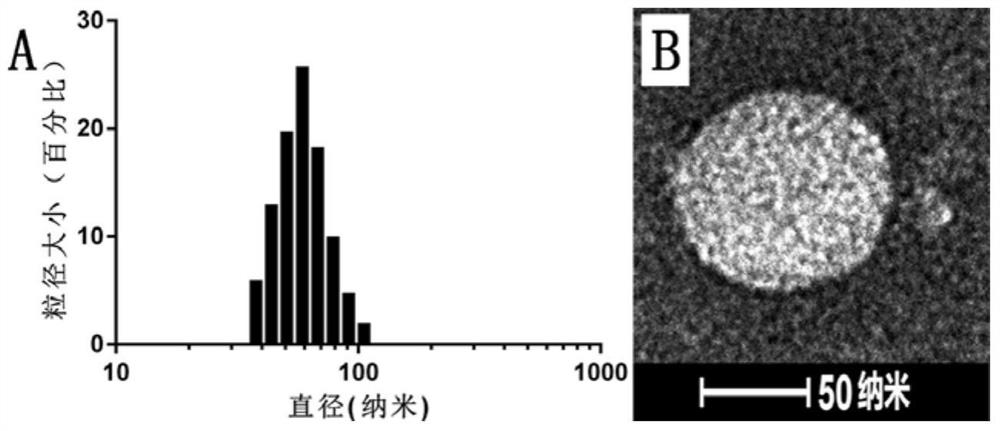
[0066] figure 1 Shown is the mass spectrogram of the PEGy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com