Production method of water-soluble monoammonium phosphate
A technology of monoammonium phosphate and production method, applied in ammonium orthophosphate fertilizer, alkaline orthophosphate fertilizer, phosphate and other directions, can solve the problem of low comprehensive utilization rate of phosphogypsum, low added value of comprehensive utilization, complicated process, etc. problems, to achieve the effect of clean processing and utilization, good economic benefits, and low process energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
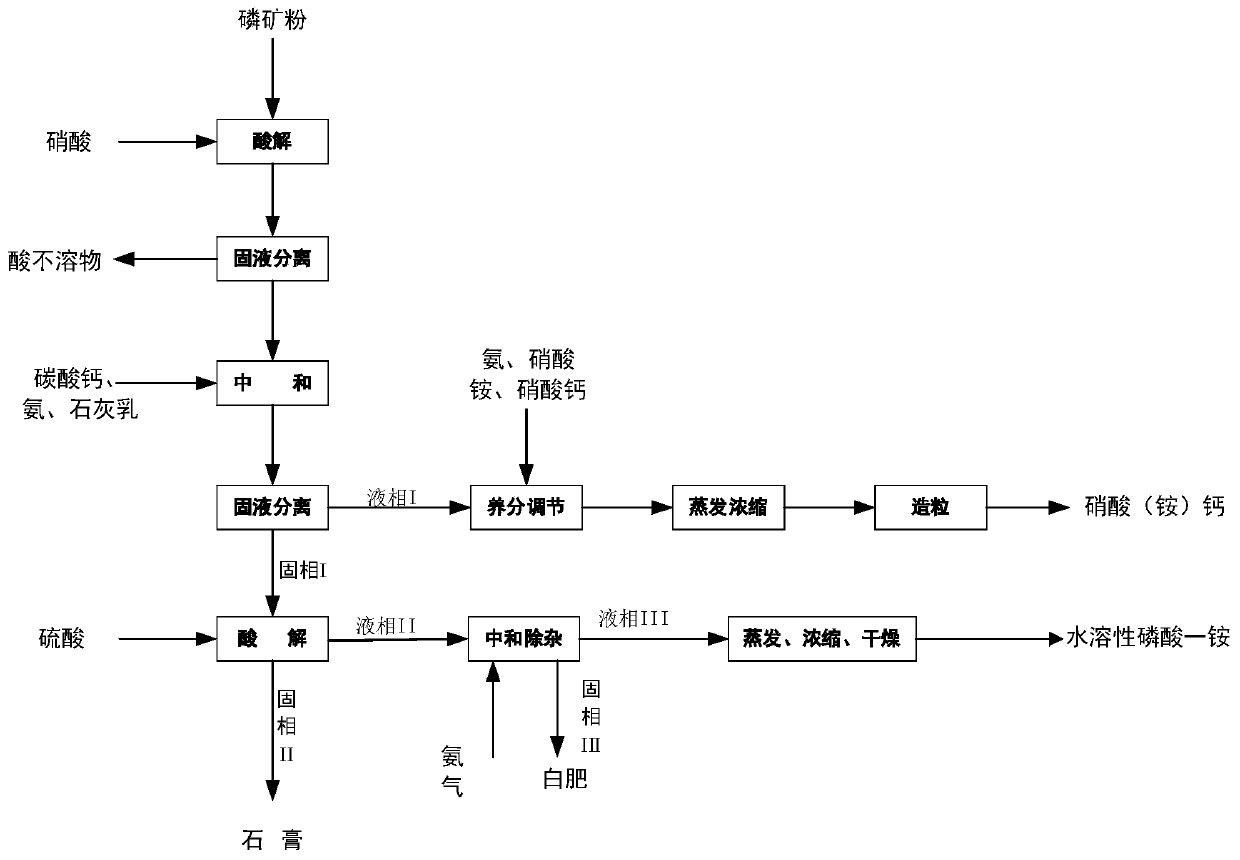
Image
Examples
Embodiment 1
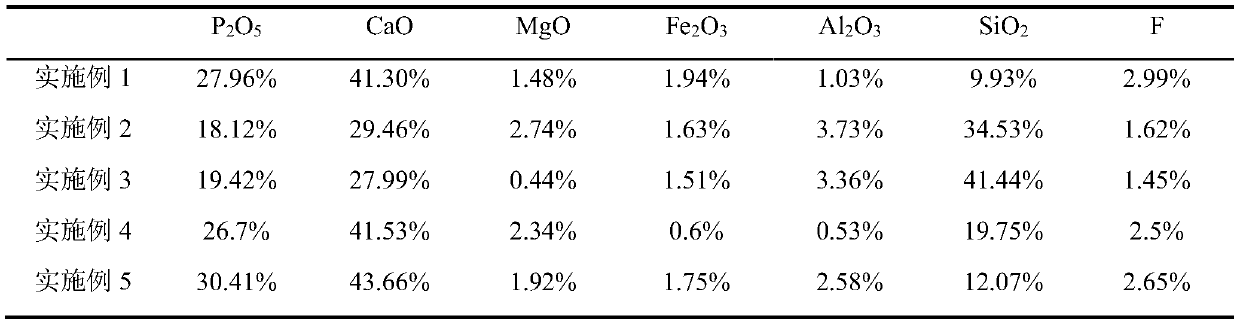
[0071] In this example, phosphate rock P 2 o 5 , CaO, MgO, Fe 2 o 3 、Al 2 o 3 , SiO 2 , F content were 27.96%, 41.30%, 1.48%, 1.94%, 1.03%, 9.93%, 2.99%, respectively, made of phosphate rock powder. The specific process is as follows:
[0072] (1) Primary acid hydrolysis: Mix phosphate rock slurry with a water content of 30% and nitric acid solution, react at 50°C for 1.5 hours to obtain a solid-liquid mixture, and obtain acid-insoluble matter and liquid phase after solid-liquid separation; among them, phosphate rock Powder is 100g, 45wt% nitric acid solution 224g; Wherein the quality of acidolysis solution is 343g, the P of acidolysis solution 2 o 5 content of 7.9%, CaO content of 11.7%;
[0073] (2) Neutralization: neutralize the liquid phase obtained in step (1) with ammonia until the solution pH=6.5; then perform solid-liquid separation to obtain liquid phase I and 65 g of solid phase I;
[0074] (3) Nutrient adjustment: the liquid phase I is mixed with an appropri...
Embodiment 2
[0080] In this example, phosphate rock P 2 o 5 , CaO, MgO, Fe 2 o 3 、Al 2 o 3 , SiO 2 , F content were 18.12%, 29.46%, 2.74%, 1.63%, 3.73%, 34.53%, 1.62%, respectively, made of phosphate rock powder. The specific process is as follows:
[0081] (1) Primary acid hydrolysis: Mix phosphate rock slurry with a water content of 20% and nitric acid solution, react at 60°C for 1 hour to obtain a solid-liquid mixture, and obtain acid-insoluble matter and liquid phase after solid-liquid separation; among them, phosphate rock powder 100g, 150g of 65wt% nitric acid solution; wherein the quality of the acid solution is 230g, the P of the acid solution 2 o 5 content of 7.8%, CaO content of 12.7%;
[0082] (2) Neutralization: neutralize the liquid phase obtained in step (1) with ammonium carbonate until the solution pH=8.0; then perform solid-liquid separation to obtain liquid phase I and 43 g of solid phase I;
[0083] (3) Nutrient adjustment: the liquid phase I is mixed with an a...
Embodiment 3
[0089] In this example, phosphate rock P 2 o 5 , CaO, MgO, Fe 2 o 3 、Al 2 o 3 , SiO 2 , F content were 19.42%, 27.99%, 0.44%, 1.51%, 3.36%, 41.44%, 1.45%, respectively, made of phosphate rock powder. The specific process is as follows:
[0090] (1) Primary acid hydrolysis: mix phosphate rock slurry with 25% water content and nitric acid solution, react at 70°C for 0.5h to obtain a solid-liquid mixture, and obtain acid-insoluble matter and liquid phase after solid-liquid separation; wherein, the phosphate rock powder is 100g, 161g of 50wt% nitric acid solution; wherein the quality of the acid solution is 235.5g, the P of the acid solution 2 o 5 content of 8%, CaO content of 11.5%;
[0091] (2) Neutralization: neutralize the liquid phase obtained in step (1) with ammonia to a solution pH=6.8; then perform solid-liquid separation to obtain liquid phase I and 44 g of solid phase I;
[0092] (3) Nutrient adjustment: the liquid phase I is mixed with an appropriate amount o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com