Peripheral thermal cross-linking dendritic group, thermal cross-linking dendritic thermal activation delayed fluorescence material as well as synthesis method and application of thermal cross-linking dendritic thermal activation delayed fluorescence material
A technology of thermally activated delayed and fluorescent materials, applied in the fields of light-emitting materials, chemical instruments and methods, electrical components, etc., can solve the problems of energy level and triplet exciton quenching problems that are difficult to be well controlled, and achieve simplified devices. Production process, good stability, low temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
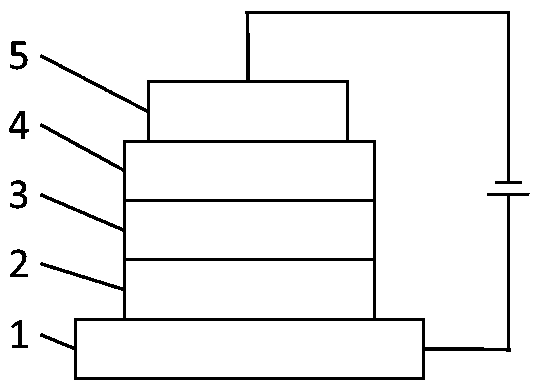
Image
Examples
Embodiment 1
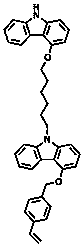
[0041] The specific reaction process of the peripheral dendron group is as follows:
[0042]
[0043] The specific synthetic method of peripheral heat-crosslinking dendron group is:
[0044] (1) At room temperature, add 4-hydroxycarbazole (5g, 27.32mmol) and 1,6-dibromohexane (26.67g, 109.28mmol) into 50mL toluene solution and stir to dissolve, then add tetrabutylammonium bromide (1 g, 3.11 mmol) and 50% NaOH (2.73 g, 68.3 mmol) in water. The reaction temperature was 60°C, and the reaction time was 2 hours. After the reaction, the crude product was purified by column chromatography to obtain the product: 9-(6-bromohexyl)-9hydrogen-4-hydroxycarbazole, with a yield of 60%. Mass Spectrum: 345.34, Elemental Analysis: The results are as follows: C: 62.44, H: 5.82, N: 4.05.
[0045] (2) Add 9-(6-bromohexyl)-9hydro-4-hydroxycarbazole (6g, 17.34mmol) from step (1) into 50mL dry N,N-dimethylformamide and stir to dissolve, Sodium hydride (0.83g, 34.68mmol) was added, and after 20 ...
Embodiment 2
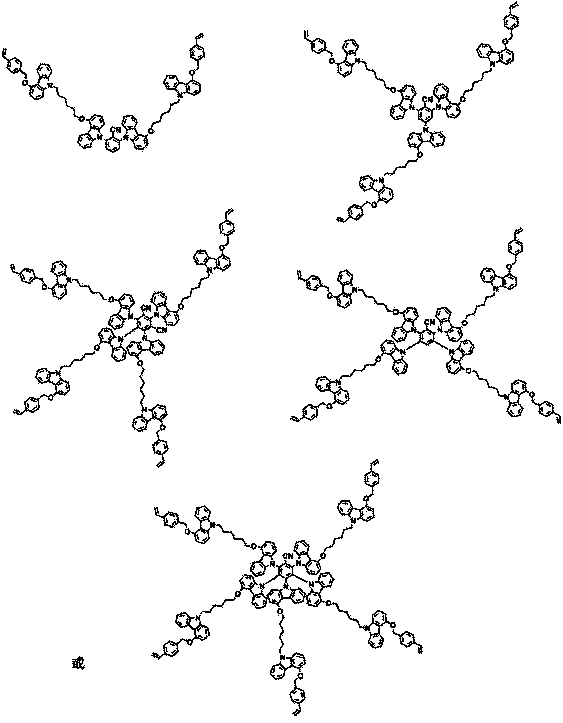
[0058] Synthesis of the target product VD2: get 9-(6-((9 hydrogen-carbazolyl-4-yl)oxyl)hexyl)-4-((4-styryl)oxyl)- in step (3) 9H-carbazole (3 g, 5.32 mmol) was dissolved in 20 mL of dry THF with stirring, and then NaH (0.26 g, 10.64 mmol) was added slowly. After reacting for 30 minutes, 2,4,6-trifluorobenzonitrile (0.21g, 1.33mmol) was added, and the reaction was carried out under the protection of nitrogen, the temperature was 60°C, and the reaction time was 24 hours; The product VD2 was purified by analytical method, yield: 78%, mass spectrum: 1791.26, elemental analysis: the results were as follows: C: 83.19, H: 6.03, N: 5.36.
[0059]
[0060] Preparation of all-wet device: The all-wet device was prepared according to the method of Example 1, except that the light-emitting layer of the device was VD2.
Embodiment 3
[0062] Synthesis of the target product VD3: get 9-(6-((9 hydrogen-carbazolyl-4-yl)oxyl)hexyl)-4-((4-styryl)oxyl)- in step (3) 9H-carbazole (3 g, 5.32 mmol) was dissolved in 20 mL of dry THF with stirring, and then NaH (0.26 g, 10.64 mmol) was added slowly. After reacting for 30 minutes, tetrafluoroisophthalonitrile (0.18g, 0.88mmol) was added, the reaction was carried out under nitrogen protection, the temperature was 60°C, and the reaction time was 24 hours; after the reaction was completed, it was purified by column chromatography to obtain Product VD3, yield: 68%, mass spectrum: 2378.89, elemental analysis: the results are as follows: C: 82.79, H: 5.93, N: 5.38.
[0063]
[0064] Preparation of all-wet device: The all-wet device was prepared according to the method of Example 1, except that the light-emitting layer of the device was VD3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com