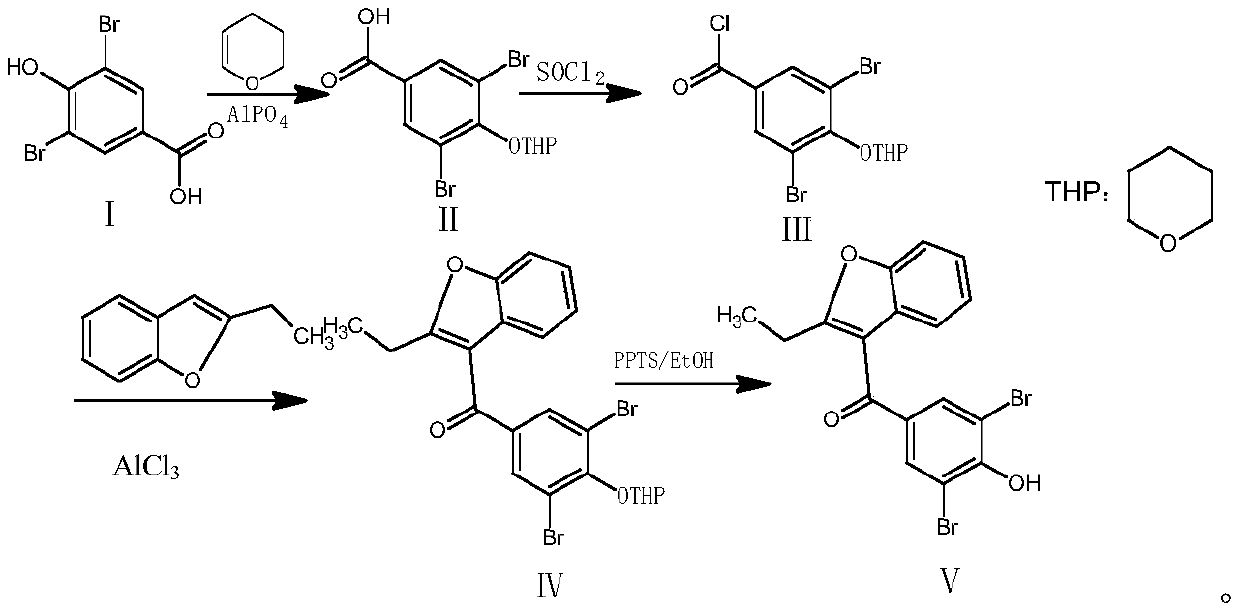
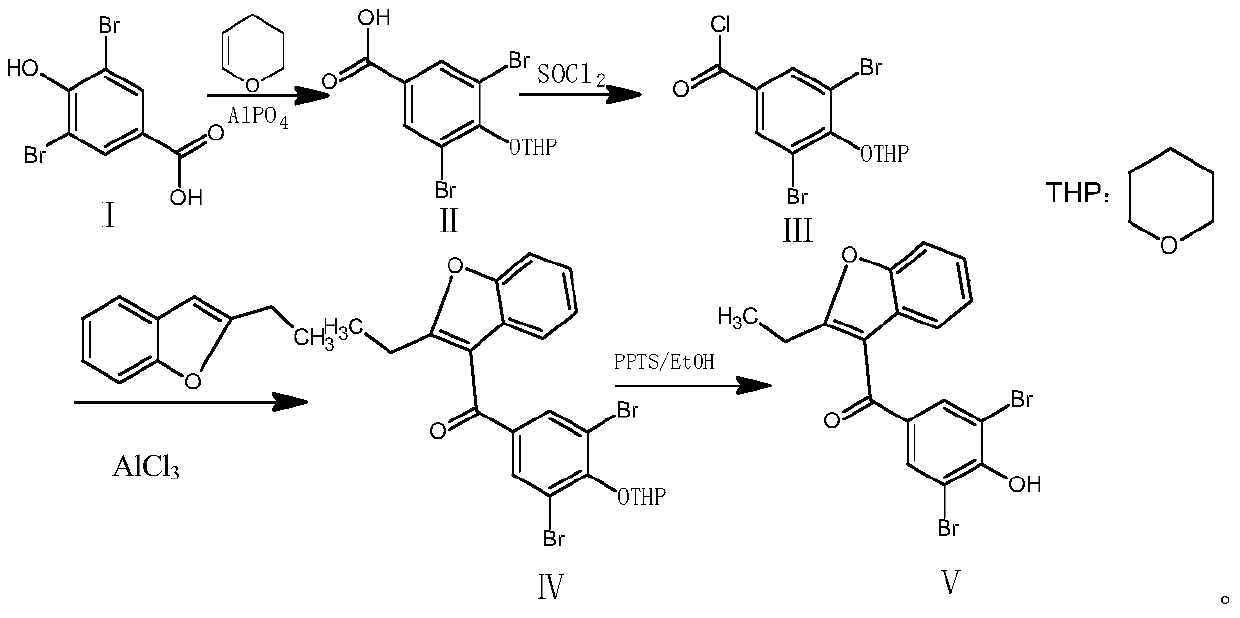
Method for preparing benzbromarone
A technology of benzbromarone and compounds, applied in the field of compound preparation, can solve problems such as easy hydrolysis and unsmooth reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of 3,5-dibromo-4-tetrahydropyranyl ether-benzoic acid (compound Ⅱ)
[0044] Add 200ml of dihydropyran, 10g of 3,5-dibromo-4-hydroxybenzoic acid, and 3g of aluminum phosphate into a 500ml reaction bottle, heat up to reflux, stir for 3 hours, cool down to room temperature, and filter off the solid Aluminum phosphate, 0.01MPa reduced pressure distillation to remove excess dihydropyran, to obtain 12.16 g of oily compound II, yield 95%, no raw material spots on TLC spot plate, TLC developer is ethyl acetate: n-hexane: acetic acid = 4: 1:0.1.
Embodiment 2
[0045] Example 2: Preparation of 3,5-dibromo-4-tetrahydropyranyl ether-benzoyl chloride (compound III)
[0046]Add 12g of the product of Example 1 into a 500ml reaction flask, add 200ml of dichloromethane, 1 drop of dimethylformamide (DMF), 8.17g of N,N-diisopropylethylamine, and stir at 15-20°C After 30 minutes, add 7.5g of thionyl chloride dropwise, and the dropping rate is controlled at an internal temperature not exceeding 25°C. After the dropwise addition is completed, keep stirring at 30-35°C for 5 hours, and filter out N,N-diisopropylethylamine. Hydrochloride, dichloromethane and excess thionyl chloride were evaporated to dryness under reduced pressure at 30°C to obtain an oily substance, which was dissolved by adding 100ml of dichloromethane, then washed and extracted by adding 100ml of saturated sodium chloride solution, and the organic layer was extracted with anhydrous sulfuric acid Magnesium was dehydrated and evaporated to dryness to obtain 12.1 g of oily compound...
Embodiment 3
[0047] Example 3: Preparation of (3,5-dibromo-4-tetrahydropyranyl ether phenyl) (2-ethyl-3-benzofuryl) ketone (compound IV)
[0048] Add 12g of compound III prepared in Example 2 into a 500ml dry reaction flask, add 200ml of dichloromethane, add 5.3g of 2-ethyl-3-benzofuran, cool down to -5°C and stir for 30min, then add 10g of aluminum trichloride, stirred and reacted for 5 hours. At this time, the color of the reaction solution changed from light yellow to dark brown. Slowly add 100ml of ice water to control the internal temperature not to exceed 5°C. The aqueous layer and the organic layer were washed twice with 100ml of saturated sodium chloride solution, then the organic layer was dehydrated by adding anhydrous magnesium sulfate, and evaporated to dryness to obtain 14g of oily compound IV with a yield of 91.5% and a purity of 96% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com