Double-antigen sandwich immunofluorescence chromatography kit for detecting African swine fever virus CD2v protein antibody
An African swine fever virus and double-antigen sandwich technology is applied in the field of veterinary biological products to achieve the effects of small variation coefficient, high detection sensitivity and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
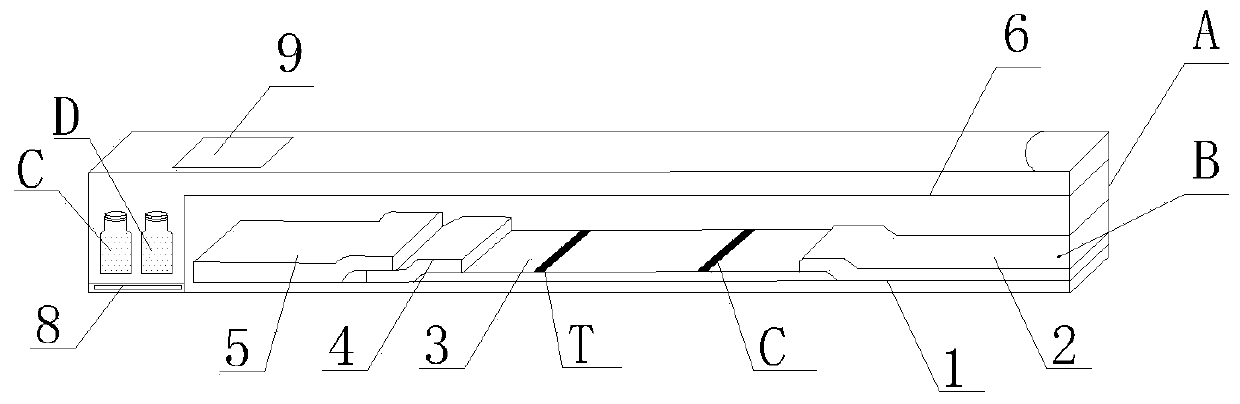
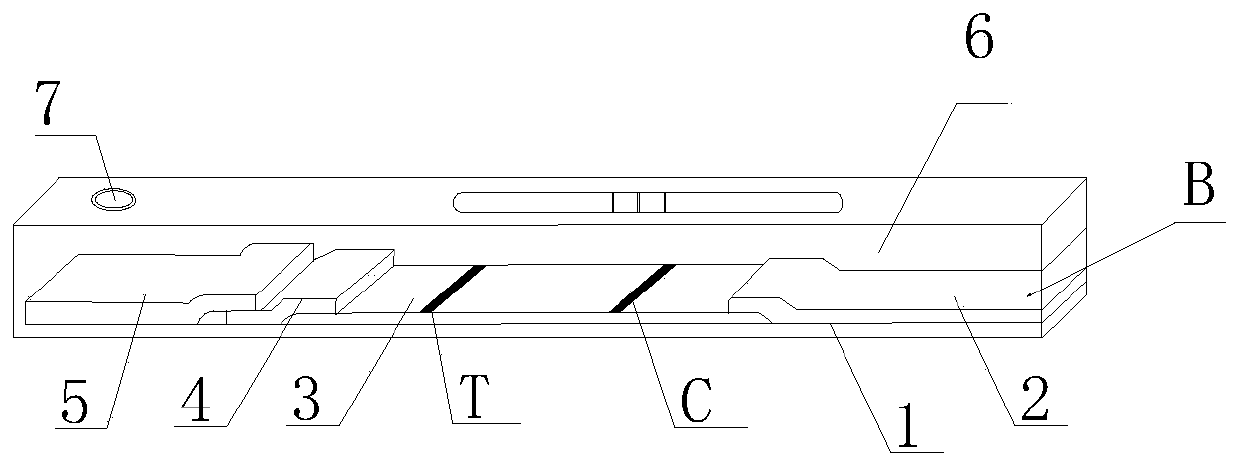
[0042] A double-antigen sandwich immunofluorescence chromatography kit for detecting African swine fever virus CD2v antibody provided by the present invention, see figure 1 , including a box body A, the box body A is equipped with a CD2v antibody immunofluorescence chromatography detection card B and a serum diluent bottle C with a serum diluent, a serum bottle D with a standard positive serum, and a standard positive serum bottle D with a standard Serum bottle E of negative serum; the CD2v antibody immunofluorescence chromatography detection card B includes a PVC bottom plate 1, and the PVC bottom plate 1 is provided with an absorption pad 2, a reaction membrane 3, a binding pad 4 and The sample pad 5, the binding pad 4 is loaded with fluorescently labeled CD2v protein and fluorescently labeled goat anti-chicken IgY, the reaction membrane 3 is provided with a detection line T and a quality control line C, the detection Line T is coated with recombinant CD2v protein, and the q...
Embodiment 2
[0046] The preparation method of the above-mentioned kit includes the following steps: flatly lay the PVC bottom plate 1 with NC film on the work surface, paste the binding pad 4, the sample pad 5 and the absorption pad 2 respectively, and cut them into 4.0mm wide strips with a strip cutter. For test strips, put each test strip into a plastic card case, place each reagent card in an aluminum film bag, add 1 pack of 1g desiccant, heat seal the seal, and it is an immunofluorescence chromatography test card.
[0047] Specifically:
[0048] 1) The coated film is made by the following method:
[0049] Dilute CD2v protein and IgY with 3% sucrose-containing PB (0.01M PH7.4) to a concentration of 1 mg / ml as the test line and quality control line solutions, draw a line on the nitrocellulose membrane, and use a marker pen at one end of the membrane Mark the quality control line and detection line. The distance between the quality control line and the detection line is 5 mm, the distanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com