Composition for type i allergy
A kind of composition, type I technology, applied in the composition of especially allergic reaction, preventing or improving the field of type I allergy, can solve the problems such as inability to guarantee safety, side effects, etc., achieve high usefulness and practicability, high safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
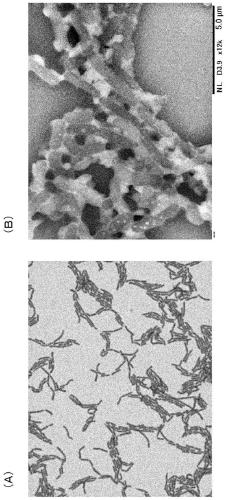
[0060] Isolation and identification of lactic acid bacteria
[0061] 1. Isolation of Lactic Acid Bacteria Samples
[0062] Select the leaves, stems and fruits of figs (variety "とよみつ姫"), use sterilized forceps and scissors to cut them into small pieces of 2-3 mm, and then add them to a sterilized medium containing MRS liquid medium. 5 to 6 fragments were placed in each test tube, and statically cultured at 28° C. and 37° C. until the MRS medium, which is a standard medium for lactic acid bacteria, became cloudy (proliferated). In addition, it took 2 to 4 days until the growth of the candidate lactic acid bacteria strain could be visually observed.
[0063] A part of each culture solution of the above-mentioned lactic acid bacteria candidate strain was streak-inoculated on the MRS agar medium with a disposable inoculating loop, and then cultured statically. Among the colonies formed on the agar medium, select all "colony colonies with different colors, luster, and shapes", and...
Embodiment 2
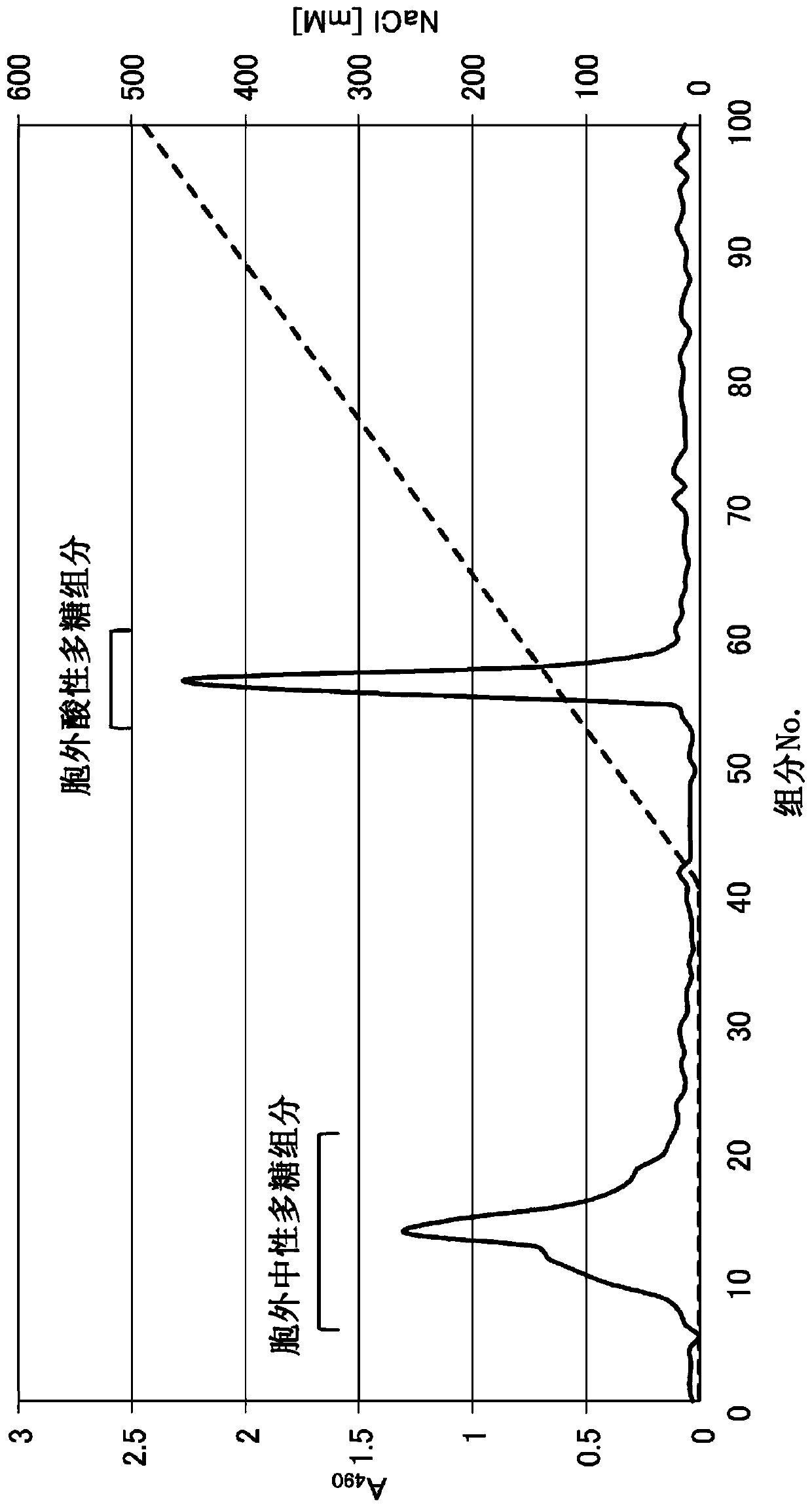
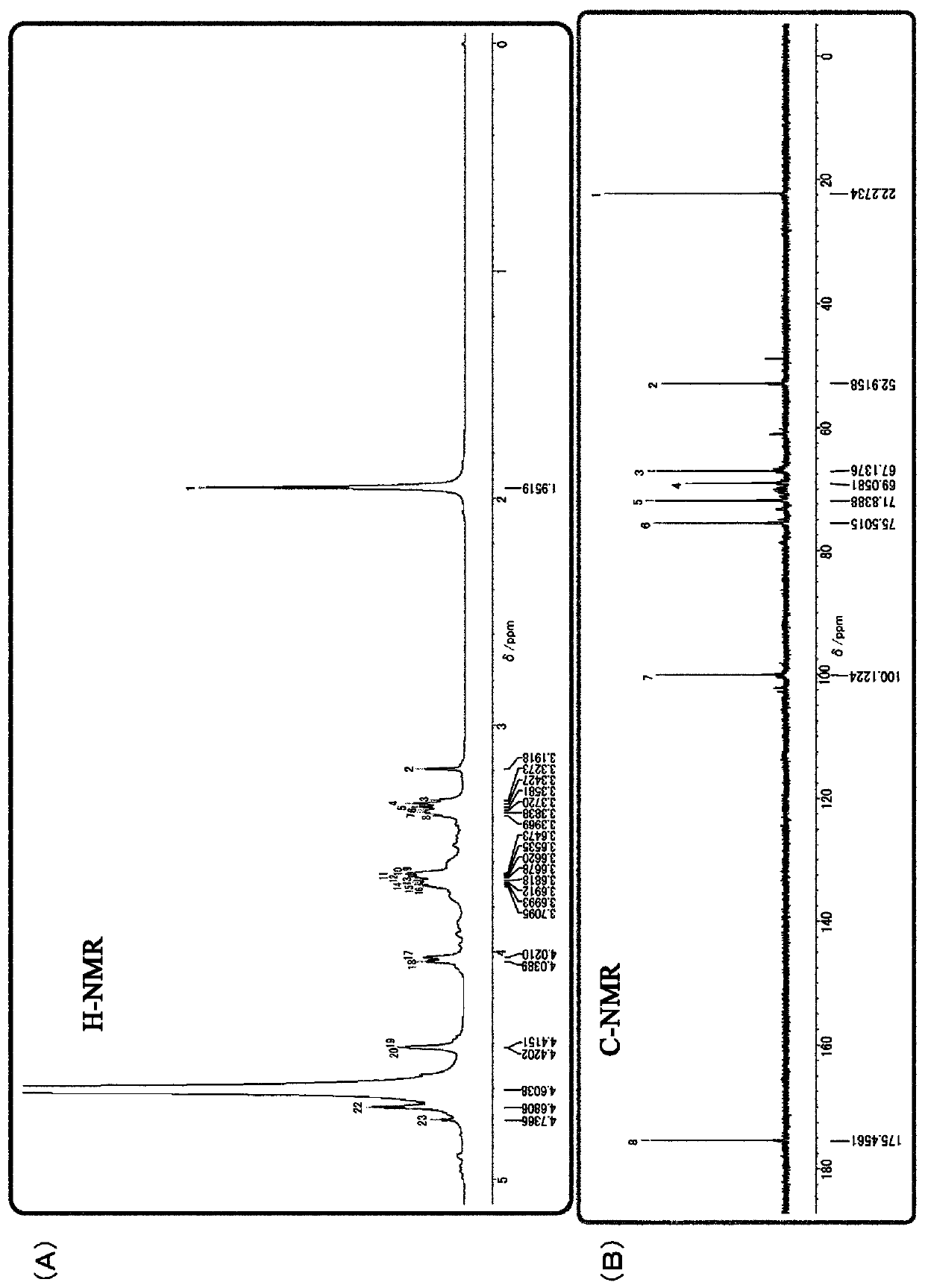
[0084] 1. Isolation and purification of polysaccharides produced by IJH-SONE68 strain
[0085] The polysaccharides produced by the IJH-SONE68 strain were isolated and purified by the following method.
[0086] The IJH-SONE68 strain was cultured statically in MRS liquid medium until the stationary phase of proliferation. 5 mL of this culture solution was inoculated into 5 L of a semi-synthetic medium for polysaccharide production (the composition will be described later) as a bacterial stock solution, and cultured at 37° C. for 120 hours. After cooling the culture solution to 4° C., 202.5 mL of 100% trichloroacetic acid aqueous solution was added to denature the protein contained in the culture supernatant and removed as a precipitate in a subsequent step, mixed and left to stand for 30 minutes. The precipitate was removed by centrifugation, an equal amount of acetone was added to the recovered supernatant, mixed, and allowed to stand overnight at 4° C., thereby precipitating ...
Embodiment 3
[0146] Inhibitory effect of IJH-SONE68 strain on active skin allergic reaction in mice
[0147] The inhibitory effect of the lactic acid bacteria of the IJH-SONE68 strain obtained in Example 1 on the active skin allergic reaction in mice was investigated according to the method described below.
[0148] 1. Test method
[0149] (1) Preparation of samples for mouse test
[0150] The Lactobacillus paracasei IJH-SONE68 strain was cultured for 48 hours in fruit juice medium (final concentration of pineapple juice: 50%, final concentration of Japanese sake extract: 10%), and the fermentation broth was concentrated 2-fold, and used as a sample for the mouse test. It is estimated that this sample contains polysaccharides including a neutral polysaccharide component and an acidic polysaccharide component at a concentration of about 40 mg / L, wherein the above-mentioned polysaccharides are polysaccharides produced by the IJH-SONE68 strain obtained in Example 2 .
[0151] (2) Reagents ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com