Traditional Chinese medicine compound preparation for treating subacute thyroiditis in acute stage and preparation method of traditional Chinese medicine compound preparation
A technology for thyroiditis and compound preparations, applied in anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., can solve the problems of syndrome differentiation, classification, and treatment of subacute thyroiditis that have not yet been formed, and achieve fewer adverse reactions, The effect of definite curative effect and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 A traditional Chinese medicine compound preparation for treating subacute thyroiditis in the acute phase and its preparation method.
[0044] A traditional Chinese medicine compound preparation for treating subacute thyroiditis, which is made of the following raw materials in parts by weight: 15 parts of Cimicifuga, 15 parts of Bupleurum, 12 parts of silkworm, 9 parts of mint, 15 parts of Coptidis, wine 15 parts of skullcap, 15 parts of burdock, 12 parts of raw licorice, 15 parts of bellflower, 15 parts of isatidis, 15 parts of forsythia, 15 parts of scrophulariaceae, 12 parts of cicada slough, and 12 parts of turmeric.
[0045] The preparation method of the traditional Chinese medicine compound preparation for treating the acute phase of subacute thyroiditis specifically includes the following steps. Step 1: Weigh Coptis chinensis, wine skullcap, burdock fruit, raw licorice, bellflower, isatidis, forsythia, crocodile, cicada slough, and turmeric in parts by w...
Embodiment 2
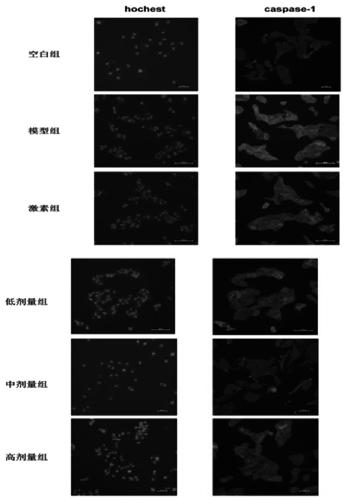
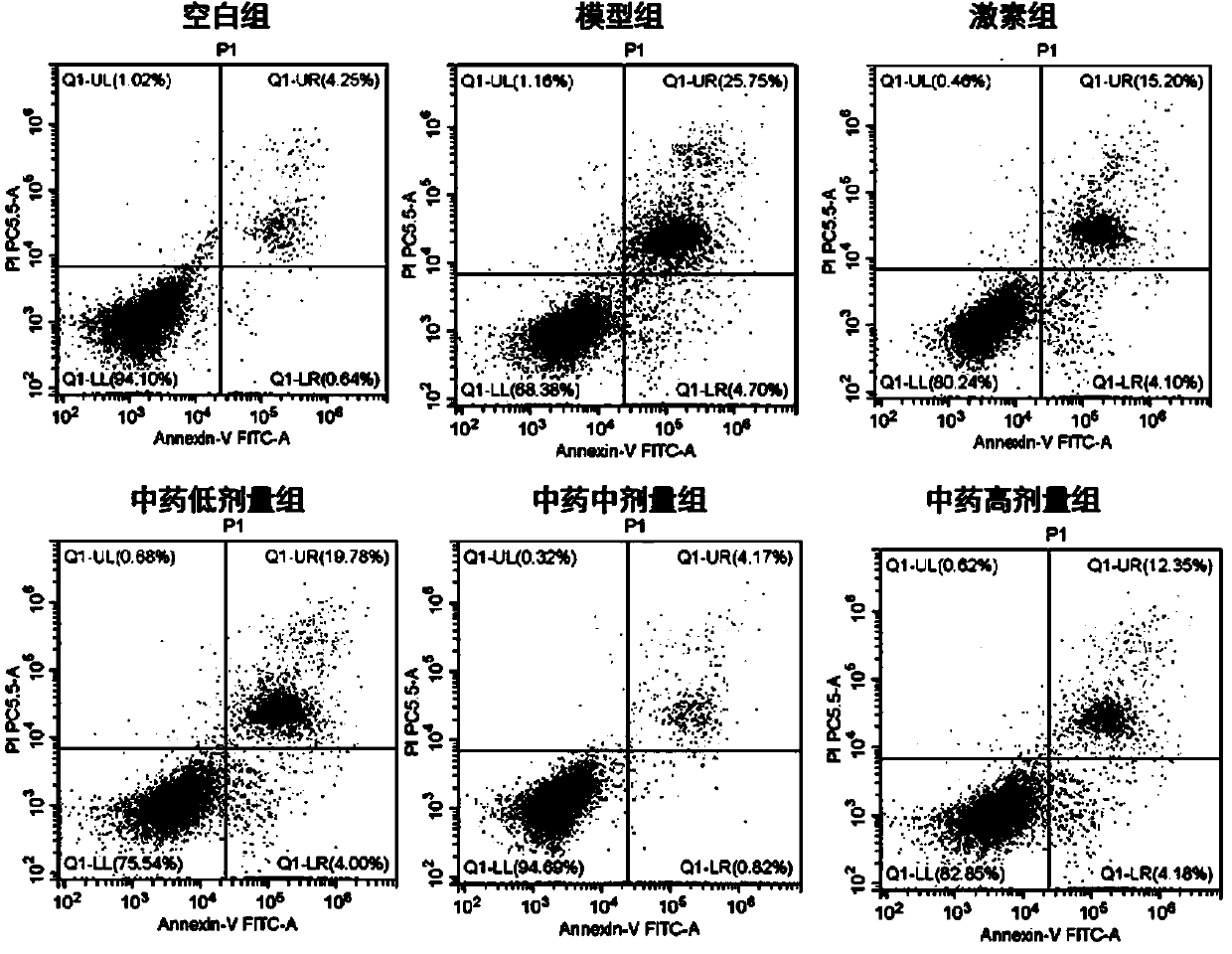
[0054] Example 2 Study on the pharmacological action mechanism of traditional Chinese medicine compound preparations for the treatment of subacute thyroiditis in the acute phase—an in vitro research experiment of interfering with the pyroptosis process of thyroid follicular epithelial Nthy-roi3-1 cells infected by adenovirus.
[0055] 1. Experimental materials.
[0056] 1.1 Main reagents.
[0057] Nthy-roi3-1, Shanghai Fuheng Biotechnology Co., Ltd.; adenovirus, Weizhen Bio; 1640 medium, Hycone Company; PBS, Hycone Company; fetal bovine serum, GIBCO Company; CytoTox Non-Radioactive Cytotoxicity Assay kit, Promega; Human Interleukin-1β ELISA Kit, Solarbio; Human Interleukin-18 ELISA Kit, Solarbio; PMSF, Beyotime; Protein Extraction Reagent (improved RIPA formula), Beyotime; Ponceau Staining solution, Beyotime; BCA protein concentration determination kit P0012, Beyotime; ultra-sensitive ECL chemiluminescence kit P0018, Beyotime.
[0058] Table 1. Quantitative Real-time PCR pr...
Embodiment 3
[0134] Example 3 clinical data.
[0135] In order to further verify the beneficial effects of the present invention, the following test cases are provided.
[0136] 1. Observation on the clinical curative effect of traditional Chinese medicine compound in treating patients with subacute thyroiditis with syndrome of flaming heat and toxin in the acute stage.
[0137] 1. Clinical data.
[0138] 1.1 General Information.
[0139] 62 patients came from newly diagnosed untreated patients with acute SAT (flashing heat and toxin syndrome) who visited the Endocrinology Department of the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine from January 2017 to December 2019. All patients were randomly divided according to the random number table. It is the traditional Chinese medicine group and the prednisone (western medicine) group, with 31 cases in each group. During the test period, 1 sample was lost in the two groups, and a total of 60 cases were included in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com