Preparation method of ratio type acidic pH fluorescent probe
A fluorescent probe, ratio-type technology, applied in the field of fluorescent probes, to achieve the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of fluorescent probes
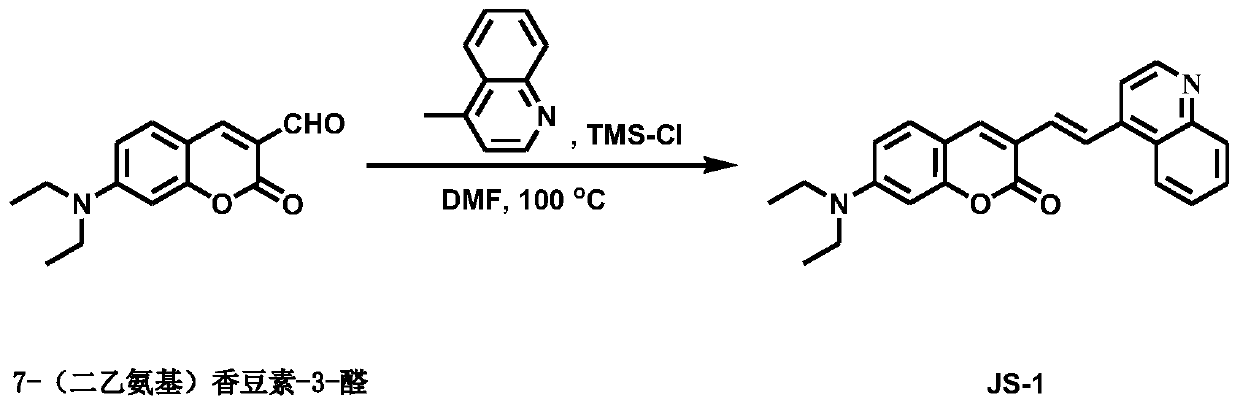
[0035] Synthetic route such as figure 1 . In a 250mL round bottom flask, dissolve 7-diethylaminocoumarin-3-aldehyde (200mg, 0.82mmol) and 4-methylquinoline (141mg, 0.98mmol) in 10ml DMF, add p-trimethyl Chlorosilane (1.1mL, 8.2mmol), wrap the bottle with aluminum foil to avoid light. in N 2 Protect and heat and stir at 100°C for 24h. Stop the reaction, after cooling to room temperature, add 100ml of water, use 1.0M Na 2 CO 3 The solution was adjusted to a solution pH of 8. Then use 100ml of dichloromethane to extract three times, collect the organic layer, and add 3g of anhydrous Na 2 SO 4 dry. The solvent was spun off, and the crude product was purified by silica gel column chromatography using petroleum ether and ethyl acetate with a volume ratio of 2:1 as the eluent, and a reddish-brown solid (100 mg, 32.9%) was obtained after purification and separation, which was the probe Molecular compound JS-1. 1 H NMR (400MHz, CDCl ...
Embodiment 2
[0037] Preparation of fluorescent probes and different pH solutions;
[0038] 1. Take an appropriate amount of probe molecule JS-1 and dissolve it in chromatographically pure acetonitrile to prepare a 1.0 mM fluorescent probe molecule stock solution.
[0039] 2. Weigh an appropriate amount of KH 2 PO 4 、Na 2 HPO 4 , NaCl, and KCl were fixed to volume with distilled water to obtain a phosphate buffer solution with a concentration of 0.01M and pH=7.4;
[0040] 3. Use 1.0M hydrochloric acid or 1.0M sodium hydroxide solution to adjust the pH of the phosphate buffer solution to obtain phosphate buffer solutions of various pHs; and according to the volume ratio of acetonitrile:PBS=4:6, prepare Acetonitrile / PBS detection solution with different pH;
[0041] 4. Add the fluorescent probe molecule stock solution in step 1 to the detection solution obtained in step 3 to obtain a mixed test solution, the concentration of the fluorescent probe molecules in the mixed test solution is 1...
Embodiment 3
[0043] UV-visible absorption spectrum determination of fluorescent probes interacting with different pH;
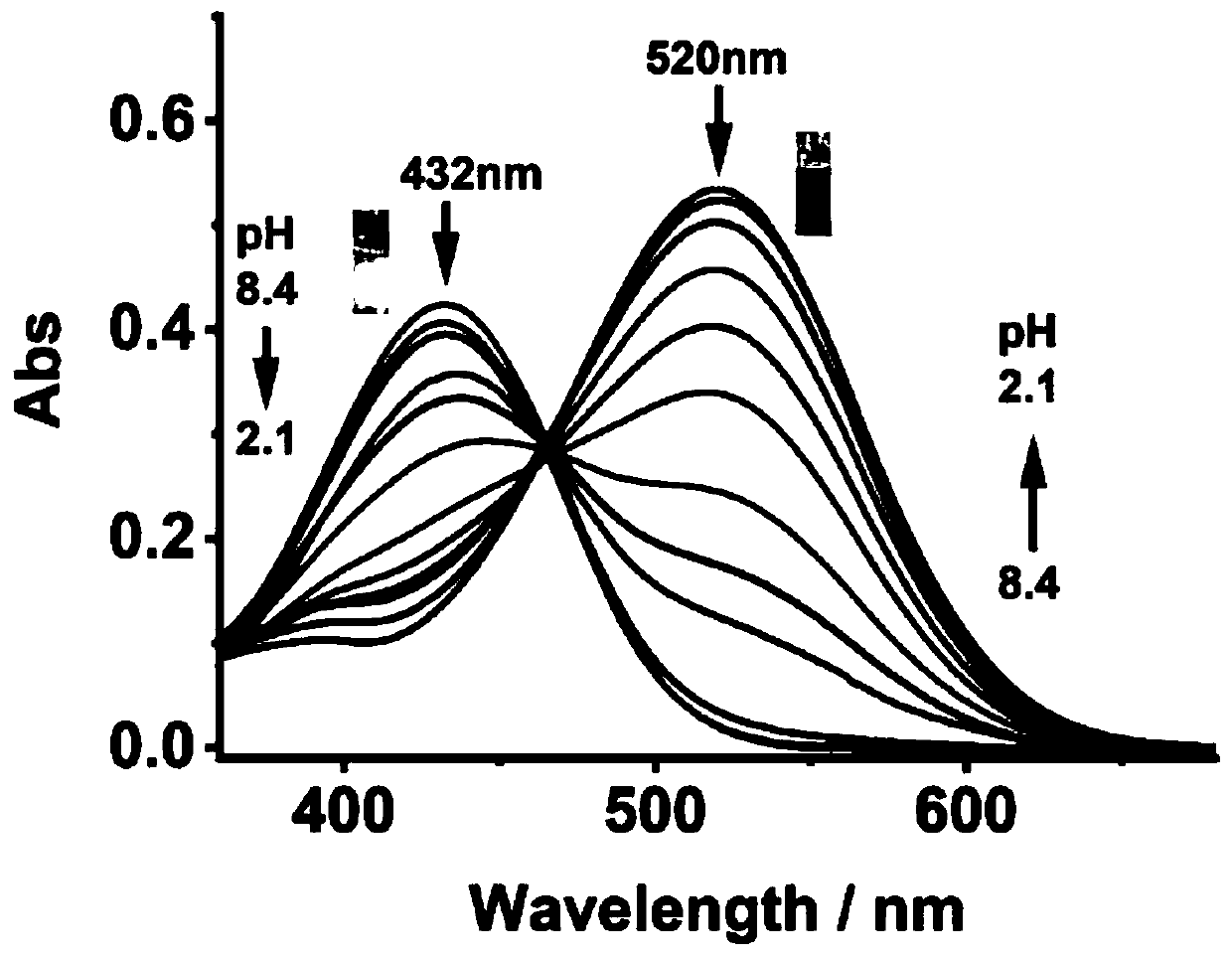
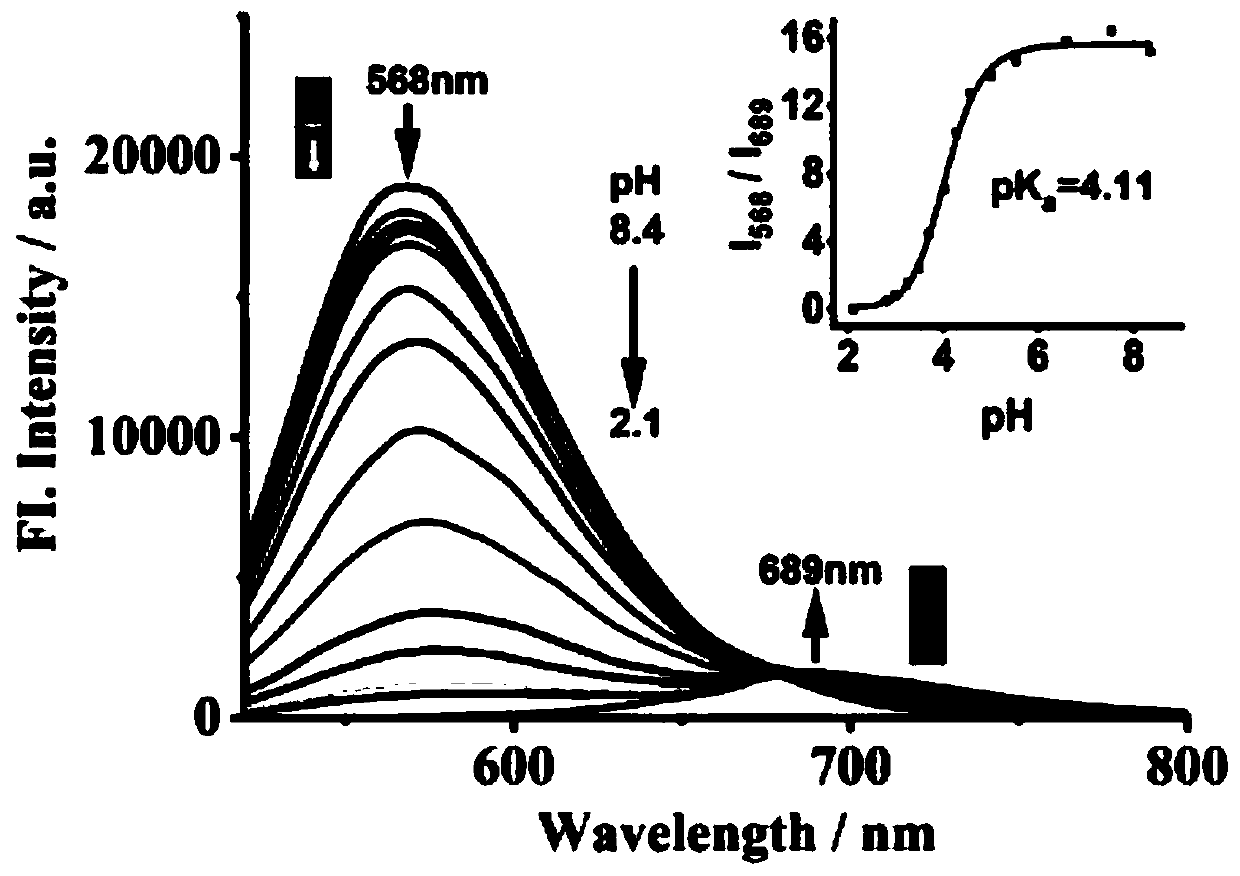
[0044] figure 2 It is the ultraviolet-visible absorption spectrum of the fluorescent probe JS-1 (10 μM) in response to different pH (2.1-8.4). The instrument used for the determination of ultraviolet-visible absorption spectrum is Shimadzu UV-2600 ultraviolet-visible spectrophotometer. Such as figure 2 As shown, when the pH is 8.4, the probe has a strong absorption at 432nm; as the pH decreases from 8.4 to 2.1, the maximum absorption wavelength of the probe red shifts to 520nm. This is due to the protonation of the N atom in the quinoline group. The color of the solution changed from light yellow (pH=8.4) to pink (pH=2.1), and the color change was obvious, which could be observed with naked eyes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com