The synthetic method of the key intermediate of tofacitinib
A synthetic method, the technology of tofacitinib, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of difficult refining of impurities, poor product purity, high cost of raw materials, etc., and achieve the effect of shortening the reaction steps, less impurities and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
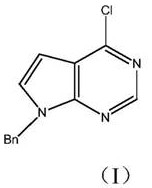
[0043] Embodiment 1: benzyl protection reaction prepares reaction product I
[0044] Starting material: 4-chloro-7H-pyrrolo[2,3-d]pyrimidine, 70g (about 0.46mol);
[0045] Organic solvent: the present embodiment is selected from acetone 350ml;
[0046] Acid binding agent I: the present embodiment is selected from sodium hydroxide 22g (about 0.55mol);
[0047] Benzyl protection reagent: this embodiment is selected from 82g (about 0.48mol) of benzyl bromide;
[0048] Benzyl protection reaction process: Add 70.0g of the initial raw material 4-chloro-7H-pyrrolo[2,3-d]pyrimidine, 350ml of acetone, 22.0g of sodium hydroxide into the reaction flask, cool down to 0-5°C, and keep warm for 0.5 h, drop 82.0g benzyl bromide into the above reaction system at 5°C, after dropping, raise the temperature to 20-23°C, and keep it warm for 3-4h; since sodium hydroxide is used as the acid-binding agent I, it can also be neutralized to form The acid promotes the reaction thoroughly, after the re...
Embodiment 2
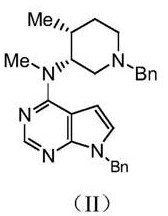
[0049] Embodiment 2: condensation reaction prepares reaction product II
[0050] Raw material 1: the reaction product I 105g (about 0.43mol) that embodiment 1 obtains;
[0051]Raw material 2: N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methylamine dihydrochloride: 113g (about 0.39mol);
[0052] Reaction solvent: water 820ml;
[0053] Acid binding agent II: the present embodiment is selected from potassium carbonate: 391g (about 2.83mol);
[0054] Condensation reaction process: add reaction solvent 820ml water, 391g potassium carbonate to the reaction flask, stir, slowly add 113g N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl Add 105g of reaction product I to amine dihydrochloride, heat to 80-110°C and reflux for 12-13h, cool down to 20-30°C and let stand for 8h, filter with suction, wash with 500ml of water, dry to obtain reaction product II, use react in the next step.
Embodiment 3
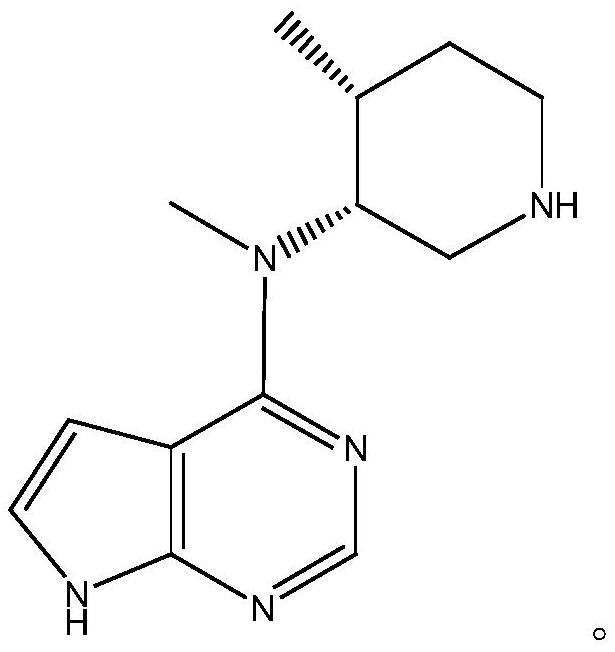
[0055] Example 3: Preparation of the product of the present invention (N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d ]Pyrimidin-4-amine (key intermediate of tofacitinib)
[0056] Raw material: the reaction product II 147g that embodiment 2 obtains;
[0057] Organic solvent: the present embodiment is selected from ethanol 1470ml;
[0058] Catalyst: the present embodiment is selected from palladium carbon 14.7g;
[0059] Reaction process for de-dibenzyl protection: add 1470ml ethanol and 147g reaction product II to the reaction bottle, after nitrogen replacement, add 14.7g palladium carbon, pass in hydrogen, raise the temperature to 60-70°C, keep the reaction for 4-5h, detect by HPLC, After the reaction is completed, cool down to 0-5°C, filter with suction, and spin to remove ethanol at 50-60°C. After spinning, stir and crystallize with acetone, filter with suction, and dry to obtain 80g of the product of the present invention; the product has a purity of 98.6%,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com