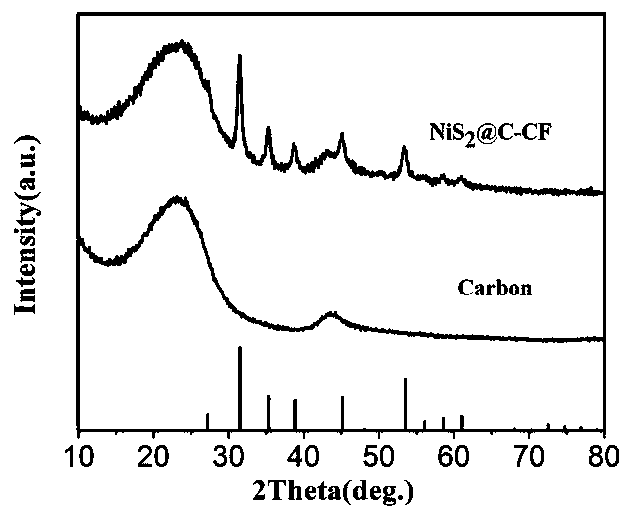
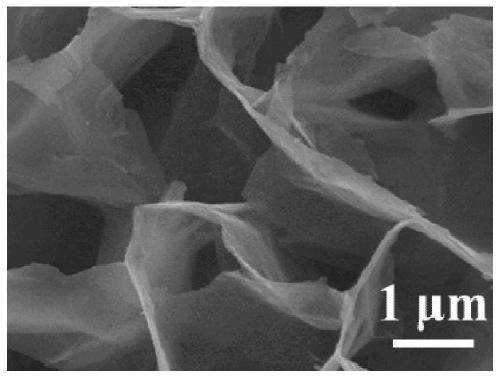
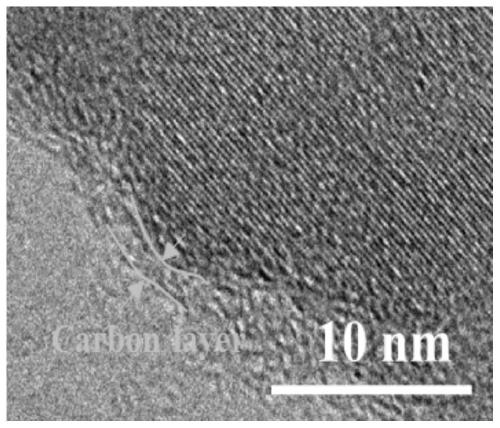
Carbon cloth-loaded carbon-coated nickel disulfide nanosheet composite material and preparation method and application thereof
A composite material, nickel disulfide technology, applied in the field of nanomaterials and electrochemistry, to achieve good cycle performance, good capacity retention, and excellent cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation method of the carbon-coated nickel disulfide nanosheet composite material supported by carbon cloth, it comprises the steps:
[0031] 1) Take 0.9 g of nickel nitrate hexahydrate and 0.56 g of hexamethylenetetramine, dissolve them in 80 ml of deionized water, and stir until completely dissolved.
[0032] 2) Put the carbon cloth into the solution obtained in step 1), and conduct a hydrothermal reaction at 100° C. for 10 h.
[0033] 3) Wash and dry the product obtained in step 2) to obtain the precursor (Ni(OH) 2 -CF).
[0034] 4) Aniline: tartaric acid: the precursor (Ni(OH) calculated in step 3) 2 -CF) was dissolved in 150ml of deionized water at a molar ratio of 3:5:3, and ultrasonicated in an ice-water bath for 30 minutes.
[0035] 5) In the solution obtained in step 4), add 0.2mol L of pre-cooled in ice water -1 Ammonium persulfate aqueous solution 50ml, the system was placed in ice-water bath for 6h.
[0036] 6) Wash the product obtained in step ...
Embodiment 2
[0041] 1) Take 2.3 g of nickel nitrate hexahydrate and 1.4 g of hexamethylenetetramine, dissolve them in 80 ml of deionized water, and stir until completely dissolved.
[0042] 2) Put the carbon cloth into the solution obtained in step 1), and conduct a hydrothermal reaction at 100° C. for 10 h.
[0043] 3) Wash the product obtained in step 2) and dry it to obtain the precursor (Ni(OH) 2 -CF).
[0044] 4) Aniline: tartaric acid: the precursor (Ni(OH) calculated in step 3) 2 -CF) was dissolved in 150ml of deionized water at a molar ratio of 3:5:3, and ultrasonicated in an ice-water bath for 30 minutes.
[0045] 5) In the solution obtained in step 4), add 0.2mol L of pre-cooled in ice water -1 Ammonium persulfate aqueous solution 50ml, the system was placed in ice-water bath for 6h.
[0046] 6) Wash the product obtained in step 5) with deionized water, and dry it in a vacuum oven at 60°C to obtain a polyaniline-coated nickel precursor composite material (Ni(OH) 2 @PANI-CF);...
Embodiment 3
[0050] 1) Take 0.9 g of nickel nitrate hexahydrate and 0.56 g of hexamethylenetetramine, dissolve them in 80 ml of deionized water, and stir until completely dissolved.
[0051] 2) Put the carbon cloth into the solution obtained in step 1), and conduct a hydrothermal reaction at 100° C. for 10 h.
[0052] 3) Wash and dry the product obtained in step 2) to obtain the precursor (Ni(OH) 2 -CF).
[0053] 4) Aniline: tartaric acid: the precursor (Ni(OH) calculated in step 3) 2 -CF) was dissolved in 150ml of deionized water at a molar ratio of 6:5:3, and ultrasonicated in an ice-water bath for 30 minutes.
[0054] 5) In the solution obtained in step 4), add 0.2mol L of pre-cooled in ice water -1 Ammonium persulfate aqueous solution 50ml, the system was placed in ice-water bath for 6h.
[0055] 6) Wash the product obtained in step 5) with deionized water, and dry it in a vacuum oven at 60°C to obtain a polyaniline-coated nickel precursor composite material (Ni(OH) 2 @PANI-CF); ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com