A nanoemulsion drug carrier and its preparation method and application
A nanoemulsion, nanoemulsion technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of short residence time and reduce the bioavailability of tacrolimus, and achieve good biocompatibility and increase solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
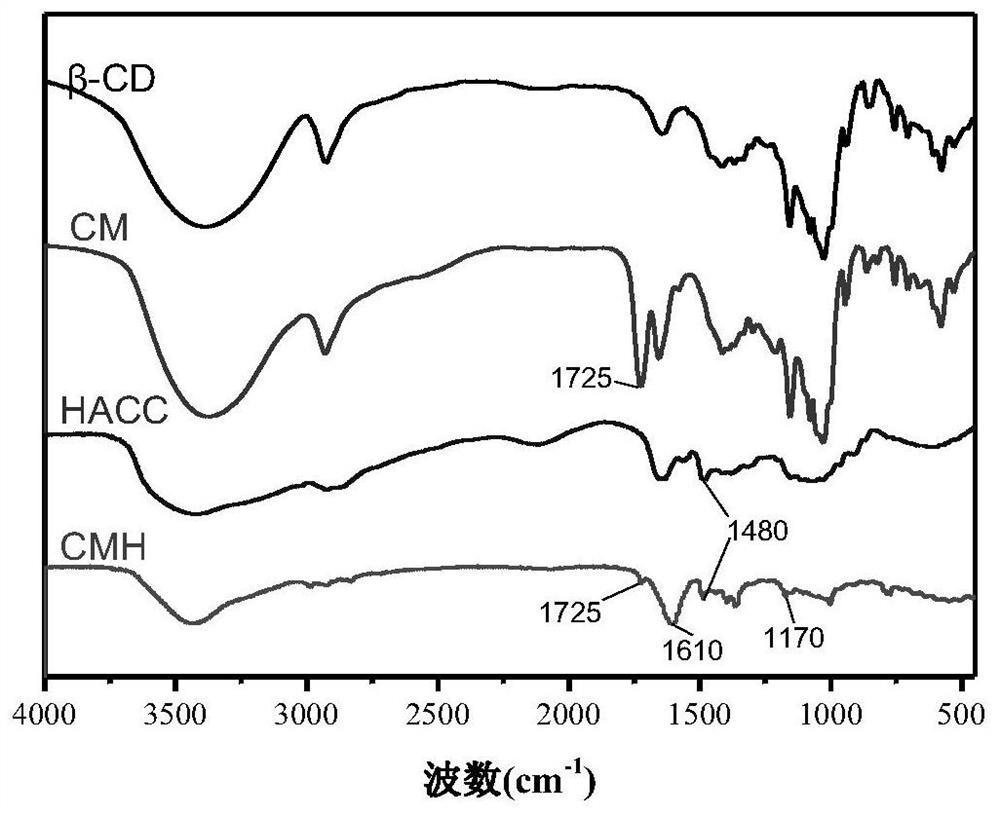
[0070] (1) Preparation of β-cyclodextrin maleic acid monoester: Dissolve 11.34g β-cyclodextrin and 9.8g maleic anhydride in 50ml N,N-dimethylformamide at room temperature , heated to 60°C to react for 8 hours, precipitated with dichloromethane and washed with acetone, collected the product by suction filtration, and dried to obtain β-cyclodextrin maleic acid monoester.
[0071] (2) Preparation of β-cyclodextrin maleic acid monoester connected with chitosan quaternary ammonium salt: β-cyclodextrin maleic acid monoester obtained by dissolving 4g of step (1) in 50ml water, adding The cross-linking agent 1g NHS and 1g EDC were activated for 2h; 3g of chitosan quaternary ammonium salt was dissolved in 50ml of water, added to the above solution under magnetic stirring, reacted at room temperature for 12h, dialyzed 3 times, each time for 12h, freeze-dried, The product is collected to obtain the quaternary ammonium salt of β-cyclodextrin maleic acid monoester connected with chitosan. ...
Embodiment 2
[0075] (1) Preparation of β-cyclodextrin maleic acid monoester: Dissolve 11.34g β-cyclodextrin and 19.6g maleic anhydride in 100ml N,N-dimethylformamide at room temperature , heated to 90°C to react for 12 hours, precipitated with dichloromethane and washed with acetone, collected the product by suction filtration, and dried to obtain β-cyclodextrin maleic acid monoester.
[0076] (2) Preparation of β-cyclodextrin maleic acid monoester connected chitosan quaternary ammonium salt: dissolve 2g of β-cyclodextrin maleic acid monoester obtained in 50ml of water in step (1), add Cross-linking agent 0.5g NHS and 1.5g EDC activation 3h; Dissolve 1.5g chitosan quaternary ammonium salt in 25ml water, add to the above solution under magnetic stirring, react at room temperature for 36h, dialyze 3 times, each 24h, Freeze-dry and collect the product to obtain the quaternary ammonium salt of β-cyclodextrin maleic butene monoester and chitosan.
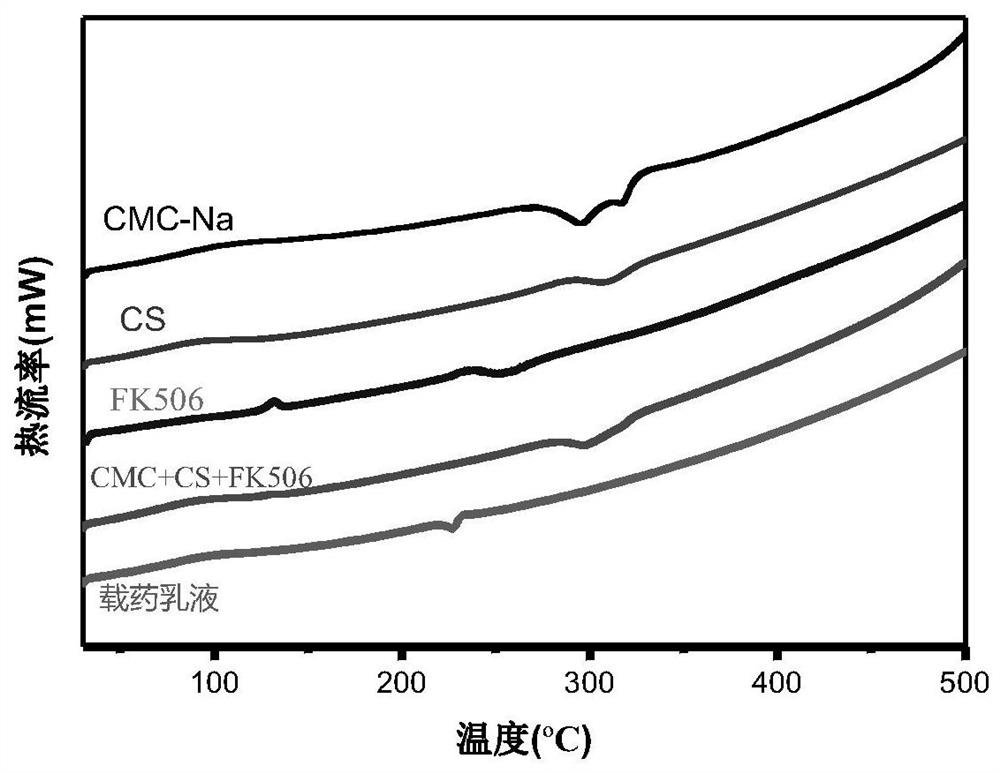
[0077] (3) Preparation of tacrolimus inclusio...
Embodiment 3
[0080] (1) Preparation of β-cyclodextrin maleic acid monoester: at room temperature, 11.34g β-cyclodextrin and 14.4g maleic anhydride were stirred in 75ml pyridine and heated to 75°C for reaction After 10 hours, precipitate with dichloromethane and wash with acetone, collect the product by suction filtration, and dry to obtain β-cyclodextrin maleic acid monoester.
[0081] (2) Preparation of β-cyclodextrin maleic acid monoester connected chitosan quaternary ammonium salt: dissolve 4g of β-cyclodextrin maleic acid monoester obtained in 50ml of water in step (1), add cross-linking 1g NHS and 1.5g EDC were activated for 2.5h; 3g chitosan quaternary ammonium salt was dissolved in 100ml water, added to the above solution under magnetic stirring, reacted at room temperature for 24h, dialyzed 3 times, each time for 18h, freeze-dried, The product is collected to obtain the quaternary ammonium salt of β-cyclodextrin maleic butene monoester and chitosan.
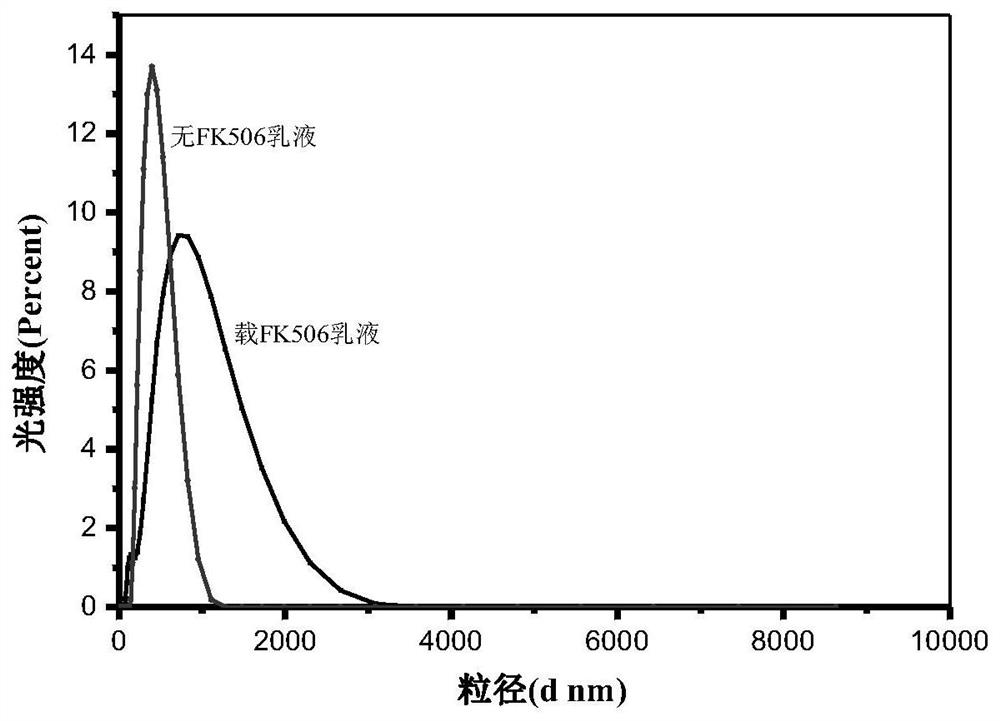
[0082] (3) Preparation of tac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com