In-vitro diagnostic kit for measuring prothrombin time
A technology of prothrombin time and in vitro diagnosis, which is applied in the direction of biological testing, material inspection products, etc., can solve the problems of difference in measurement results, difficulty in comparison of results, poor sensitivity, etc., and achieve improved stability, good stability, and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
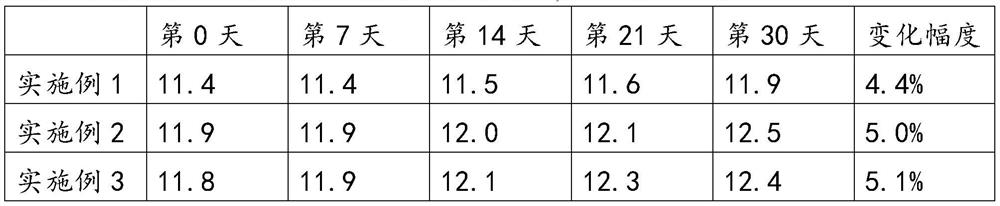
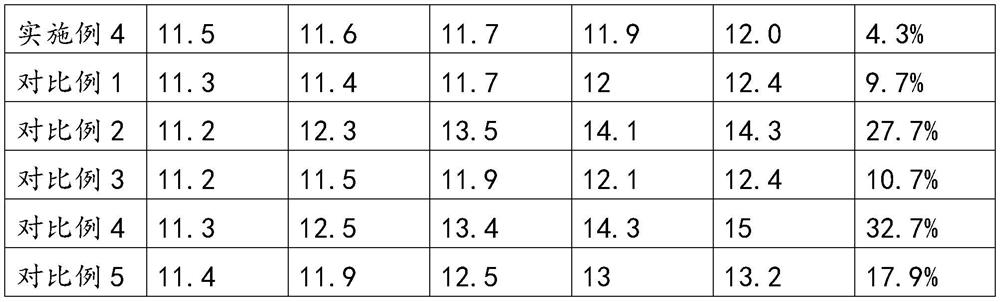
[0047] An in vitro diagnostic kit for measuring prothrombin time, comprising a prothrombin time measuring reagent and a plastic bottle for holding the prothrombin time measuring reagent; wherein, the prothrombin time measuring reagent consists of The buffer system is composed of thromboplastin, and the thromboplastin is extracted from rabbit brain powder; the buffer system is made of the following raw materials: buffer, calcium ion reagent, surfactant, amino acid, trehalose and proclin300; in the buffer solution system, the molar concentration of the buffer agent is 0.2M, the mass fraction of the calcium ion reagent is 8%, the mass fraction of the surfactant is 5%, the mass fraction of the amino acid The mass fraction of the trehalose is 0.3%, the volume fraction of the proclin300 is 0.3%, and the pH value of the buffer solution system is 7.0-8.0.
[0048] The buffer is selected from Tris-Hcl buffer; the calcium ion reagent is calcium chloride; the surfactant is polyethylene g...
Embodiment 2
[0051] An in vitro diagnostic kit for measuring prothrombin time, comprising a prothrombin time measuring reagent and a plastic bottle for holding the prothrombin time measuring reagent; wherein, the prothrombin time measuring reagent consists of The buffer system is composed of thromboplastin, and the thromboplastin is extracted from rabbit brain powder; the buffer system is made of the following raw materials: buffer, calcium ion reagent, surfactant, amino acid, trehalose and proclin300; in the buffer solution system, the molar concentration of the buffer agent is 0.01M, the mass fraction of the calcium ion reagent is 0.5%, the mass fraction of the surfactant is 0.1%, and the mass fraction of the amino acid The mass fraction of the trehalose is 0.1%, the volume fraction of the proclin300 is 0.1%, and the pH value of the buffer solution system is 7.0-8.0.
[0052] The buffer is selected from MPOS buffer; the calcium ion reagent is calcium chloride; the surfactant is sodium do...
Embodiment 3
[0055] An in vitro diagnostic kit for measuring prothrombin time, comprising a prothrombin time measuring reagent and a plastic bottle for holding the prothrombin time measuring reagent; wherein, the prothrombin time measuring reagent consists of The buffer system is composed of thromboplastin, and the thromboplastin is extracted from rabbit brain powder; the buffer system is made of the following raw materials: buffer, calcium ion reagent, surfactant, amino acid, trehalose and proclin300; in the buffer solution system, the molar concentration of the buffer agent is 0.5M, the mass fraction of the calcium ion reagent is 15%, the mass fraction of the surfactant is 10%, the mass fraction of the amino acid -15%, the mass fraction of trehalose is 0.5%, the volume fraction of proclin300 is 0.5%, and the pH value of the buffer solution system is 7.0-8.0.
[0056] The buffer is selected from HEPES buffer; the calcium ion reagent is calcium chloride; the surfactant is triton; and the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com