Preparation method of 4,4',4''-tritert-butyl-2,2':6',2''-terpyridine
A technology of tert-butyl pyridine and tributyl tin alkyl pyridine, which is applied in the field of organic synthesis, can solve the problems of low synthesis yield, difficult product separation and purification, use of high-risk reagents, etc., and achieves the effect of simple separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of Compound IV:
[0034]
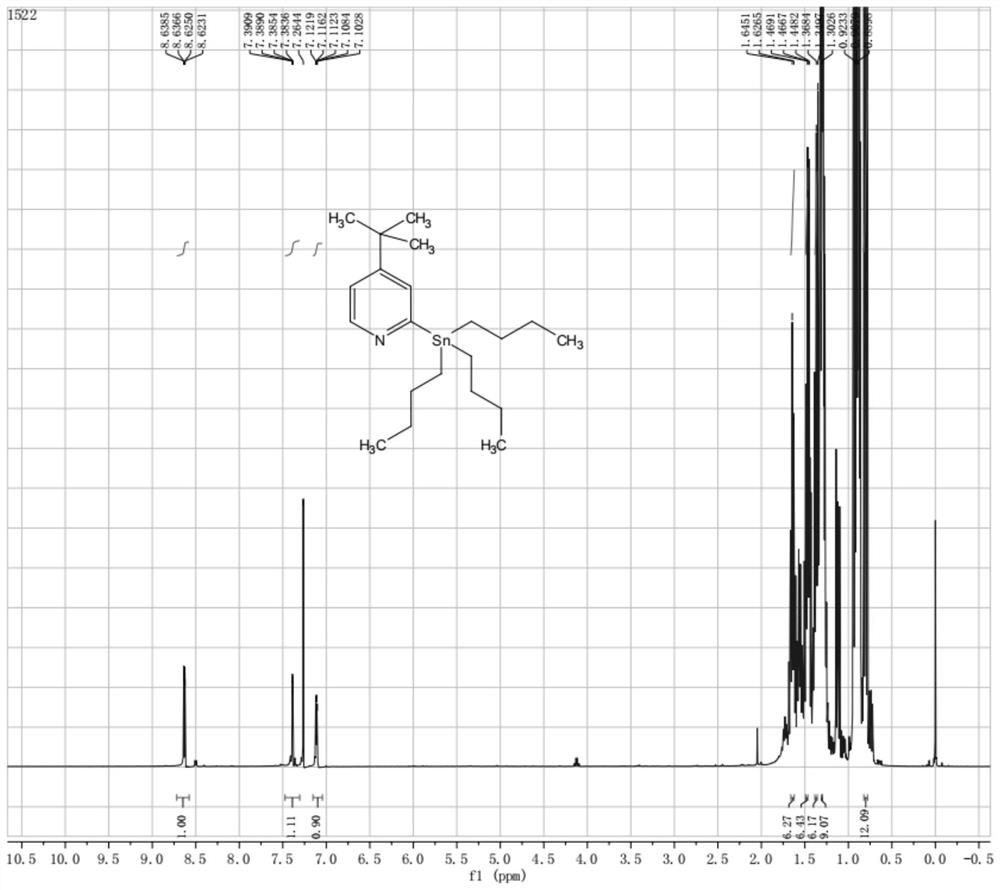
[0035] Under the protection of nitrogen, N,N-dimethylethanolamine (131.5 g, 1.48 mol, 2.0 equiv.) was added into 2.5 L of n-hexane, and the temperature was lowered to -20°C. Add n-butyllithium (2.5 M in hexanes, 1.18 L, 2.96 mol, 4.0 equiv.), react at -30°C for 30 minutes, and then add 4-tert-butylpyridine (100.0 g, 0.74 mol, 1.0 equiv. ), react at -20°C for 1 hour after the dropwise addition is completed. Cool the above reaction solution to -70°C, add tributyltin chloride (481.7 g, 1.48 mol, 2.0 equiv.) dropwise, react at -70°C for 2 hours after the dropwise addition, naturally rise to room temperature, and detect the reaction by TLC Finish. The reaction solution was added with 1.0 L of ice water, extracted with ethyl acetate (500 mL), washed with water, column chromatography, and quickly rinsed with petroleum ether to obtain 210.97 g of a colorless liquid, yield: 67.2%, purity 85%.
[0036] 1 H NMR (400 MHz, CDCl 3 ) δ (ppm...
Embodiment 2
[0039] Synthesis of Compound IV:
[0040]
[0041] Under nitrogen protection, N,N-dimethylethanolamine (44.57 g, 0.50 mol, 2.0 equiv.) was added to 1.0 L of n-heptane, and the temperature was lowered to -20°C. Add sec-butyllithium (1.3 M in hexanes, 0.77 L, 1.0 mol, 4.0 equiv.), react at -30°C for 30 minutes, and then add 4-tert-butylpyridine (33.80 g, 0.25 mol, 1.0 equiv. ), react at -20°C for 1 hour after the dropwise addition is completed. Cool the above reaction solution to -70°C, add tributyltin chloride (97.65 g, 0.30 mol, 1.2 equiv.) dropwise, react at -70°C for 2 hours after the dropwise addition, naturally rise to room temperature, and detect the reaction by TLC Finish. The reaction solution was added to 500 mL of ice water, extracted with ethyl acetate (500 mL), washed with water, column chromatography, and quickly eluted with petroleum ether to obtain 40.83 g of a colorless liquid, yield: 38.5 %.
Embodiment 3
[0043] Synthesis of compound I:
[0044]
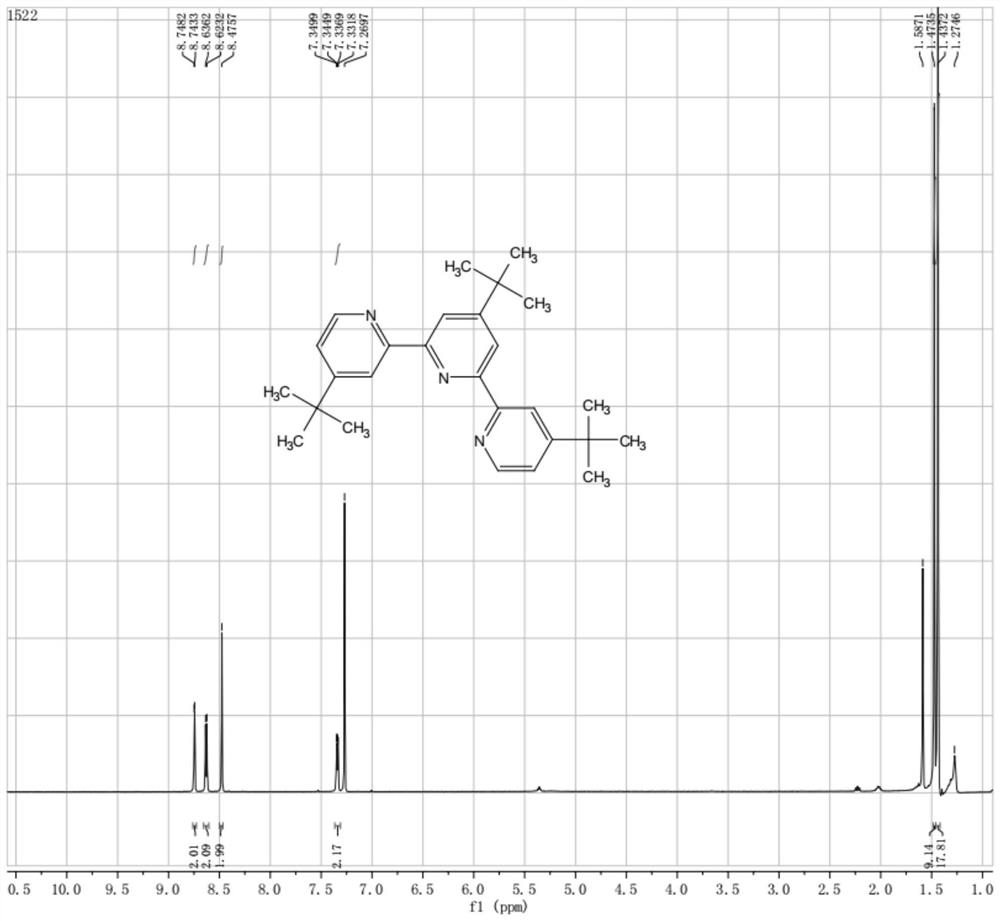
[0045]Under nitrogen protection, 4-tert-butyl-2-tributylstannylpyridine (IV, 50.00 g, 0.118 mol, 2.0 equiv., theoretical) prepared in Example 1, 4-tert-butyl-2,6-di Chloropyridine (V, 13.24 g, 0.065 mol, 1.1 equiv.), Pd(dppf)Cl 2 (2.16 g, 3.0 mmol, 5 mol%) was added into 100 mL of toluene, heated to 100°C for 16 hours, and the TLC reaction was complete. The reaction solution was concentrated under reduced pressure, made into sand, and column chromatography (washed with DCM) to obtain 17.06 g of a white solid with a yield of 72.0% and a purity of 97%.
[0046] 1 H NMR (400 MHz, CDCl3) δ (ppm): 8.74 (d, J = 1.96 Hz, 2H), 8.63 (d, J = 5.2Hz, 2H), 8.48 (s, 2H), 7.34 (dd, J = 5.2, 2.0 Hz, 2H), 1.47 (s, 9H), 1.44 (s, 18H).
[0047] LC-MS (ESI+): m / z 423.95 (M+Na).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com