Novel coronavirus (SARS-COV-2) spike protein binding molecule and applications thereof
A SARS-COV-2 and spike protein technology, applied in the field of medicine and biology, can solve the problems of being difficult to become a special drug for SARS-COV-2, limited therapeutic effect, etc., and achieve good specificity, inhibition of infection and amplification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Screening of single domain antibodies against SARS-COV-2-Spike protein
[0096] 1.1 Library construction
[0097] 1.1.1 Immunity
[0098] The alpaca was immunized with the Spike-RBD protein of the new coronavirus, and immunized at the 1st, 2nd, 4th, and 6th week, respectively, for a total of 4 immunizations, and each immunization dose was 200ug.
[0099] 1.1.2 Extraction of total RNA
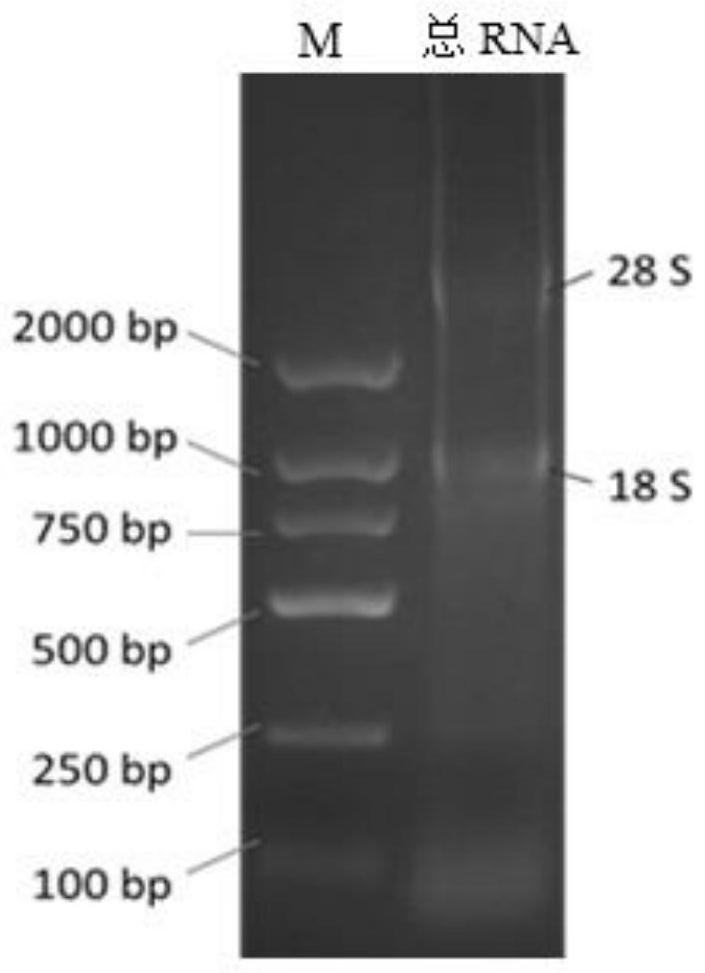
[0100] Take 50ml of alpaca peripheral blood after immunization in the sixth week, separate lymphocytes, extract total RNA of lymphocytes with Trizol, and use UV spectrophotometer to detect the extracted RNA results: OD260 / 280=1.99, OD260 / 230=1.43 , indicating that the extracted RNA was not significantly degraded, and the purity was good; the total RNA concentration was 809.3ng / μL. The extracted total RNA was used for agarose gel electrophoresis, and the results were as follows: figure 1 As shown, two bands of 28S and 18S can be seen.
[0101] 1.1.3 RNA reverse transcription
[0102]...
Embodiment 2
[0151] 1.1 Preparation of Fc fusion protein of SARS-COV-2-Spike protein single domain antibody
[0152] According to the amino acid sequence of the constant region of human immunoglobulin (IgG1) on the protein database Uniprot, the amino acid sequence of the human IgG1-Fc region (SEQ ID NO: 109) was obtained. Obtain the nucleic acid fragment (nucleic acid sequence such as SEQ ID NO: 137) encoding human IgG1-Fc from human PBMC total RNA by reverse transcription PCR, and then obtain the fusion protein of SARS-COV-2-Spike protein single domain antibody and Fc by overlapping PCR The encoding nucleic acid fragment was recombined into the vector pCDNA4 (Invitrogen, CatV86220).
[0153] The successfully constructed pCDNA4 plasmid containing the nucleic acid fragment of the fusion protein of SARS-COV-2-Spike protein single domain antibody and Fc was transfected into HEK293 cells for expression. Specifically, the recombinant expression plasmid was diluted with Freestyle293 medium and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com