Method for improving expression level of human coagulation factor IX
A technology of human blood coagulation factor and expression level, applied in the field of biochemistry, can solve the problems of large economic burden, high treatment cost, iron element accumulation, etc., achieve the effect of reducing dosage, reducing production cost, and improving expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
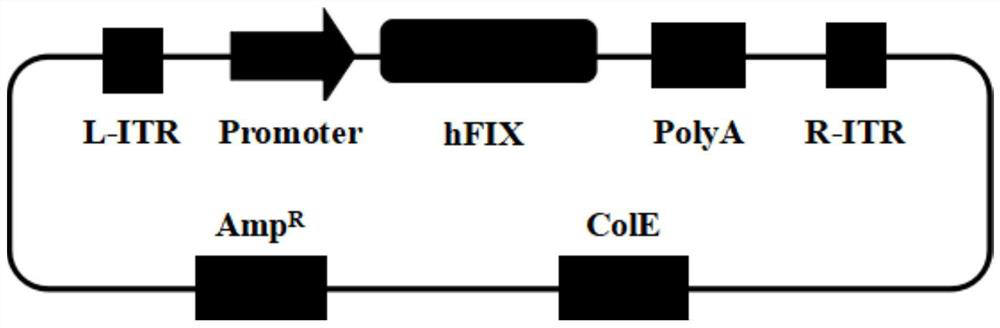
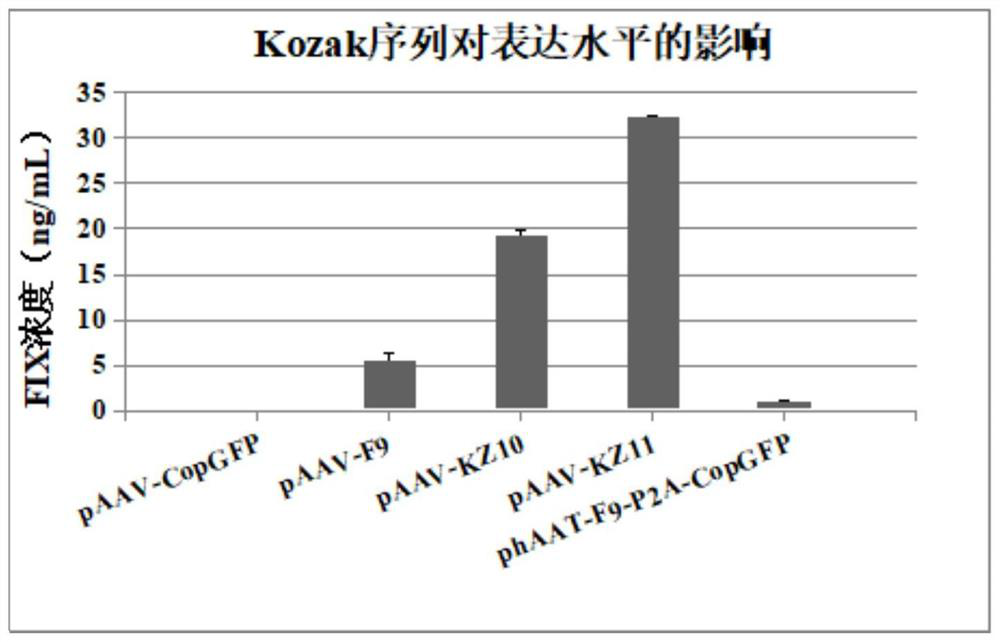
[0024] Replacement of Kozak sequence improves the expression of FIX
[0025] 1) Synthetic FIX gene
[0026] DNA synthesis is entrusted to a third-party company. The synthesized sequence includes: promoter, 5' UTR, the optimized FIX gene, 3' UTR, polyA tailed signal sequence, and NotI added at both ends of the sequence Enzyme recognition site gcggccgc. After sequencing, the sequence is completely correct.
[0027] 2) Linearization of FIX expression sequence and AAV expression vector
[0028] The reaction system is:
[0029] DNA 1 μg
[0030] 10×Buffer 5μL
[0031] NotI restriction endonuclease 1 μL
[0032] wxya 2 O supplemented to 50 μL
[0033] The reaction conditions are:
[0034] 37℃, 1-16h
[0035] 3) Fragment recycling:
[0036] The reaction solution after digestion was subjected to agarose gel electrophoresis, and the agarose concentration was 1%. Electrophoresis parameters are: voltage 120V, time 30min. Cut out the target fragment under UV light. Recover u...
Embodiment 2
[0099] High expression of plasmid-mediated hFIX in HEK293 cells
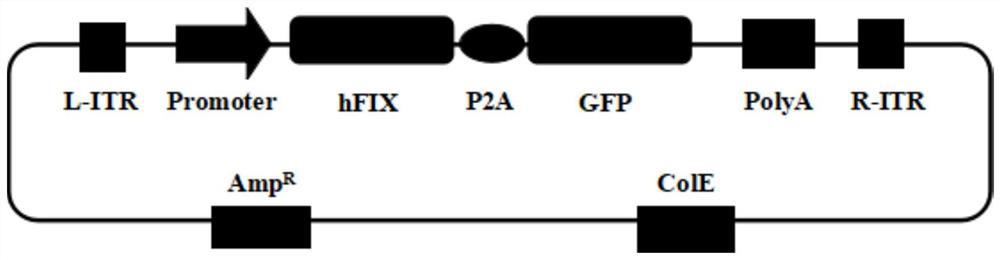
[0100] 1) Construction of pCMV-F9-P2A-GFP plasmid
[0101] The green fluorescent protein GFP was cloned after the FIX gene on the above-mentioned AAV expression plasmid pAAV-F9, and the stop codon TAG of the FIX gene was removed at the same time, and the P2A sequence ggaagcggagctactaacttcagcctgctgaagcaggctggagaacgtggaggagaaccctggacct was added between the FIX gene and the GFP gene. The promoter of the above-mentioned AAV expression plasmid was replaced with the CMV promoter. The obtained plasmid was named pCMV-F9-P2A-GFP. For a schematic diagram of the plasmid see image 3 ;
[0102] 2) Transfection
[0103] HEK293T cells were seeded in 24-well plates at a cell density of 10 5 One per well, 12-16h later, transfection was performed using Lipo3000 transfection reagent. 4-6h after transfection, replace the new medium. 48h after transfection, the fluorescence of the cells could be observed under a fluorescenc...
Embodiment 3
[0107] Recombinant adeno-associated virus (AAV) mediated FIX gene to highly express human blood coagulation factor FIX in HepG2
[0108] 1) Packaging and purification of recombinant AAV virus
[0109] A three-plasmid packaging system was adopted, including the above-mentioned AAV expression plasmid pAAV-F9, the helper plasmid pHelper and the packaging plasmid pRC2 / 8.
[0110] HEK293T cells were seeded in five 15cm culture dishes. After 12-18 hours, the confluence of the cells reached 85-90%, and the cells were in good condition. The medium was replaced with serum-free DMEM medium 1-2 hours before transfection.
[0111] Prepare DNA-PEI complex: Add 124μg pHelper plasmid, 76μg pRC2 / 8 plasmid and 65.1μg pAAV-F9 plasmid to 5mL DMEM medium in turn, vortex and mix well; add 1ml PEI with a concentration of 1mg / mL to 5mL DMEM In the culture medium, vortex to mix; add PEI solution to the plasmid solution, vortex to mix, and stand at room temperature for 20 min. Evenly drop the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com