Quality detection method of Jindan pills
A quality detection method, the technology of Jindan, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the mutual interference between qualitative and quantitative, the quality control of Jindan pills, the difficulty of quality inspection, and the inability to meet the simultaneous qualitative determination of the chemical components of Jindan pills and quantitative problems, to achieve the effect of good repeatability and accurate quality detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The present embodiment is the quality detection method of Jindan pill, and the steps are as follows:
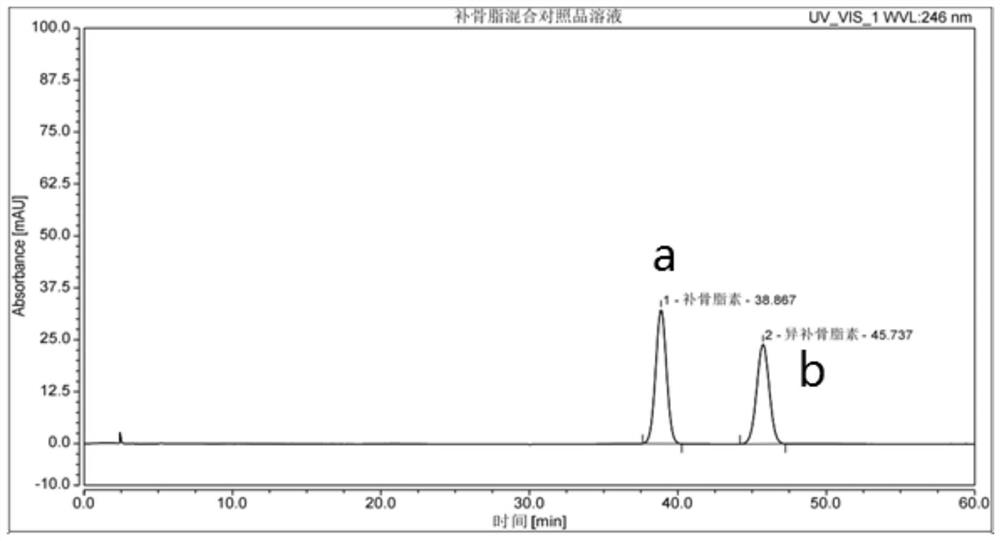
[0058] (1) Chromatographic conditions and adaptability test: the chromatographic column is CAPCELL PAK C18 4.6*250mm 5μm, which uses octadecyl bonded silica gel as filler; methanol-water (volume ratio 32:68) as mobile phase; detection The wavelength is 246nm; the column temperature is 30°C; the flow rate is 1.0ml / min; the number of theoretical plates should not be less than 3000 based on the psoralen peak.
[0059] (2) Preparation of reference substance solution: Accurately weigh psoralen and isopsoralen reference substances, put them in a volumetric flask, add methanol to dilute to the mark, and make each 1mL containing 0.02mg of psoralen, isopsoralen Mixed solution of 0.02 mg of osteolipin, shake well, and obtain.
[0060] (3) Preparation of the test solution: get 14g of Jindan Pill sample (source Guangzhou Baiyunshan Qixing Pharmaceutical Co., Ltd., batch number K8...
Embodiment 2
[0066] This embodiment is a condition investigation experiment of the quality detection method of Jindan pills described in Example 1.
[0067] 1. The influence of the detection wavelength on the detection results
[0068] Preparation of the test solution: take Jindan Pills for the test (sourced from Guangzhou Baiyunshan Qixing Pharmaceutical Co., Ltd., batch number K8001A), grind, accurately weigh 1.5g, place in a stoppered Erlenmeyer flask, and accurately add Methanol 25mL, weighed, heated to reflux for extraction for 2 hours, allowed to cool, weighed again, made up for lost weight with methanol, shaken well, filtered, and obtained the filtrate.
[0069] Chromatographic conditions: chromatographic column is CAPCELL PAK C18 4.6*250mm 5μm, which uses octadecyl bonded silica gel as filler; methanol-water (volume ratio 32:68) as mobile phase; column temperature 30°C; flow rate 1.0ml / min. Precisely draw 10 μL of the Jindan Pill test solution for injection and detection. Absor...
Embodiment 3
[0118] This embodiment is a methodological investigation of the quality detection method of Jindan pills described in Example 1.
[0119] 1. Specific inspection
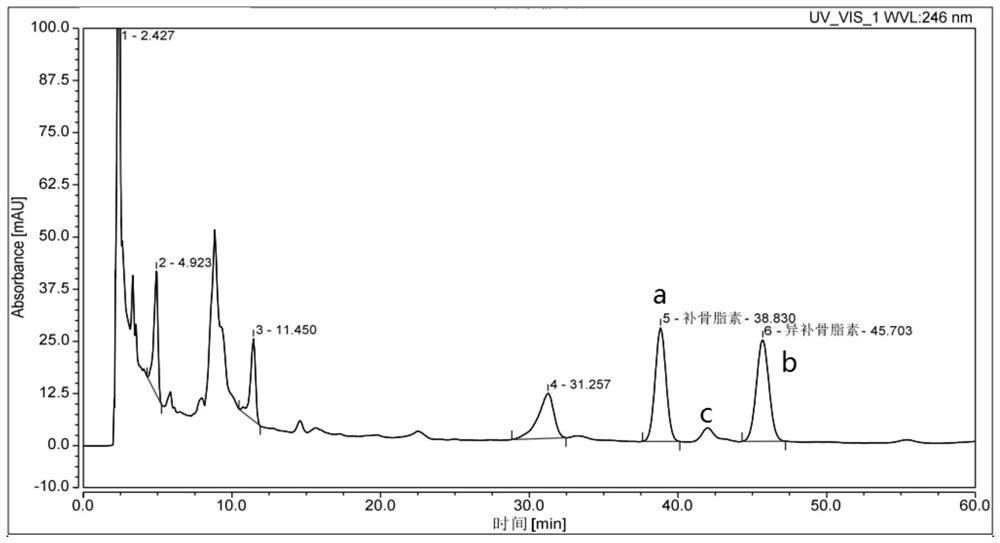
[0120] Prepare the negative reference substance not containing psoralen according to the same preparation process as Jindan Pills, and prepare the negative sample solution according to the same method of "preparation of the test solution" item in Example 1, and then take the blank Solvent (methanol), Jindan pill need testing solution, measure respectively by the same chromatographic condition of " chromatographic condition and adaptability test " item in embodiment 1, the results see Image 6 , Figure 7 with Figure 8 . It can be seen that the blank solvent and negative sample spectrum have the same retention time position as the psoralen and isopsoralen reference substances, and no peaks appear, indicating that the blank solvent and negative sample have no interference to the determination, and the method has st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com