Salbutamol sulfate inhalation preparation and preparation method thereof
A technology of albuterol sulfate and inhalation preparations, which is applied in the direction of medical formulas, respiratory diseases, and medical preparations of non-active ingredients, etc., which can solve the problems of reduced drug compliance, bitter taste of erythromycin, and unacceptable children, and achieve a reduction Toxic and side effects, eliminate fear, improve the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
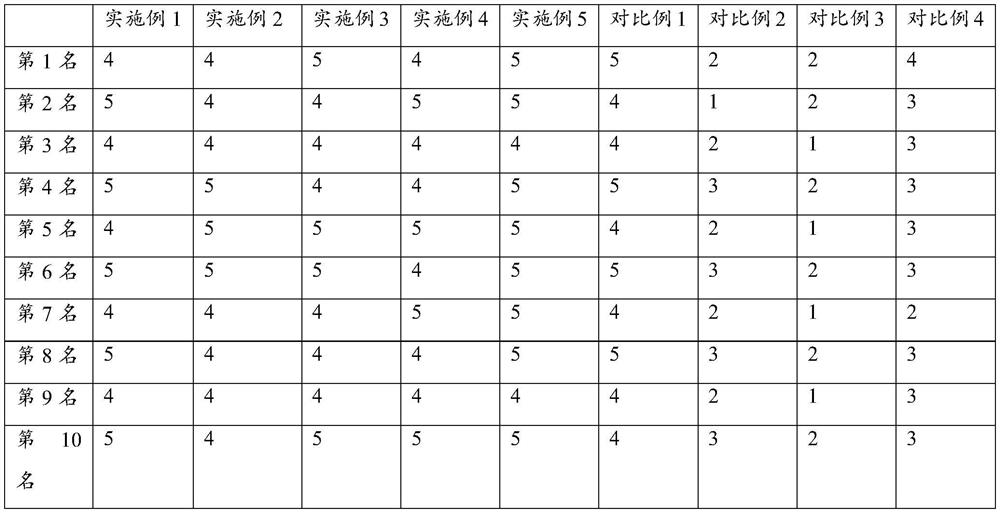
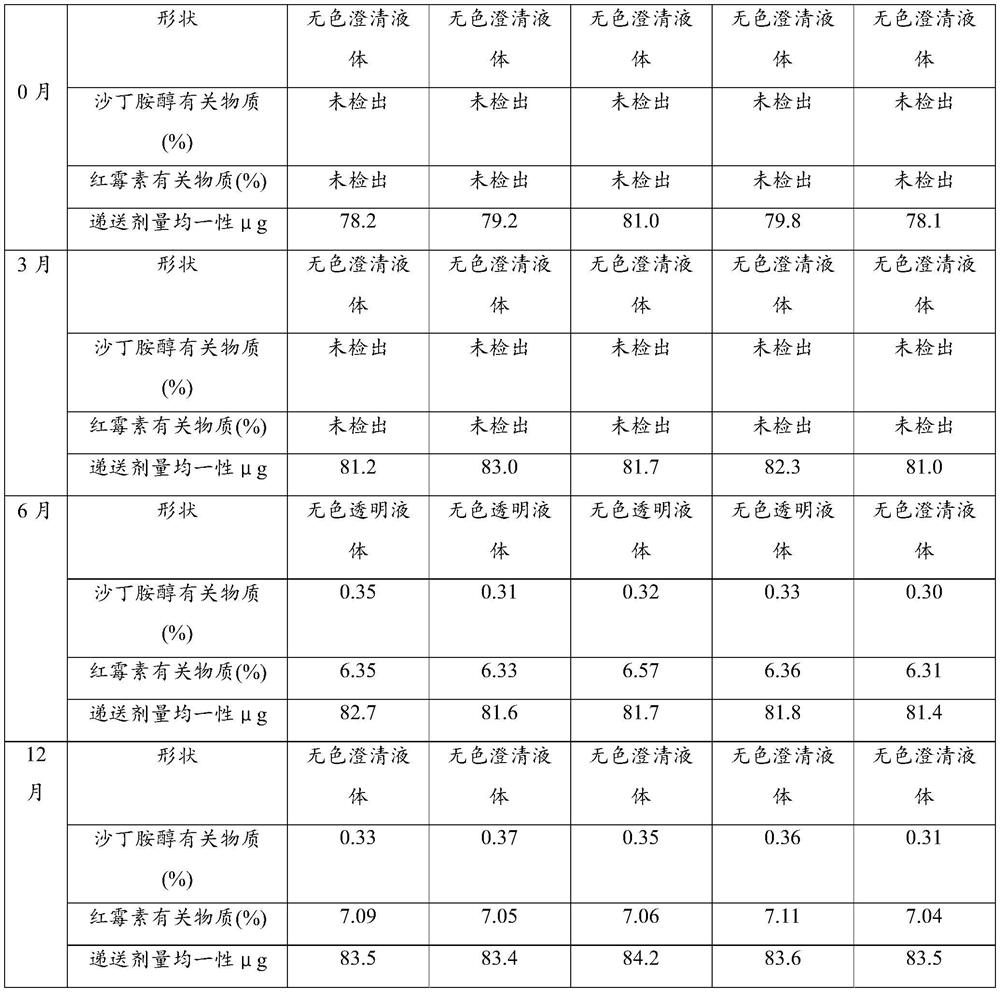
Examples
Embodiment 1
[0029] Embodiment 1, salbutamol sulfate inhalation preparation of the present invention and preparation method thereof
[0030] formula:
[0031] Salbutamol Sulfate 1.5g
[0032] Erythromycin 0.1g
[0033] L-fucose 0.3g
[0034] Taste masking agent 2g
[0036] 8% hydrochloric acid solution 2g
[0037] Make 1000ml;
[0038] Wherein the taste-masking agent comprises maltodextrin and polyethylene glycol-600, and the mass ratio of maltodextrin and polyethylene glycol-600 is 1:0.5;
[0039] The preparation method comprises the following steps:
[0040] S1. Dissolve the prescribed amount of maltodextrin in water for injection, add the prescribed amount of salbutamol sulfate, erythromycin, L-fucose, polyethylene glycol and osmotic pressure regulator, fill with nitrogen and stir to dissolve, the stirring speed 800rpm / min, stirring time is 30min, cooled to room temperature, to obtain a mixed solution;
[0041] S2. Regulate the pH value of the mix...
Embodiment 2
[0043] Embodiment 2, salbutamol sulfate inhalation preparation of the present invention and preparation method thereof
[0044] formula:
[0045] Salbutamol Sulfate 1.8g
[0046] Erythromycin 0.3g
[0047] L-fucose 0.2g
[0048] Taste masking agent 1g
[0049] Glucose 5g
[0050] Citric Acid-Sodium Citrate 2g
[0051] Make 1000ml;
[0052] Wherein the taste-masking agent comprises maltodextrin and polyethylene glycol-600, and the mass ratio of maltodextrin and polyethylene glycol-600 is 1:0.6;
[0053] The preparation method comprises the following steps:
[0054]S1. Dissolve the prescribed amount of maltodextrin in water for injection, add the prescribed amount of salbutamol sulfate, erythromycin, L-fucose, polyethylene glycol and osmotic pressure regulator, fill with nitrogen and stir to dissolve, the stirring speed 600rpm / min, stirring time is 30min, cooled to room temperature, to obtain a mixed solution;
[0055] S2. Use a pH regulator to adjust the pH value of th...
Embodiment 3
[0057] Embodiment 3, albuterol sulfate inhalation preparation of the present invention and preparation method thereof
[0058] formula:
[0059] Salbutamol Sulfate 0.5g
[0060] Erythromycin 0.2g
[0061] L-fucose 0.4g
[0062] Taste masking agent 2.5g
[0063] Glucose 8g
[0064] Citric Acid - Disodium Hydrogen Citrate 2g
[0065] Make 1000ml;
[0066] Wherein the taste-masking agent comprises maltodextrin and polyethylene glycol-600, and the mass ratio of maltodextrin and polyethylene glycol-600 is 1:0.7;
[0067] The preparation method comprises the following steps:
[0068] S1. Dissolve the prescribed amount of maltodextrin in water for injection, add the prescribed amount of salbutamol sulfate, erythromycin, L-fucose, polyethylene glycol and osmotic pressure regulator, fill with nitrogen and stir to dissolve, the stirring speed 900rpm / min, stirring time is 30min, cooled to room temperature, to obtain a mixed solution;
[0069] S2. Use a pH regulator to adjust the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com