Method for producing phenylenediamine by solvent-free hydrogenation continuous reaction of dinitrobenzene
A technology for producing phenylenediamine and dinitrobenzene, which is applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problem of reducing the yield of phenylenediamine, high material consumption and energy consumption, and many side reactions to achieve significant economic and social benefits, reduce production material consumption and energy consumption, and reduce by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A method for producing phenylenediamine by continuous hydrogenation reaction of dinitrobenzene without solvent, using dinitrobenzene as raw material to prepare phenylenediamine through continuous hydrogenation reaction, comprising the steps of:
[0049] S1, 100 grams of water, 100 grams of phenylenediamine (wherein, the weight ratio of o-phenylenediamine, m-phenylenediamine and p-phenylenediamine is 1:0.7:0.7, that is, 40 grams of o-phenylenediamine, M-phenylenediamine is 30 grams, and p-phenylenediamine is 30 grams), and 10 grams of Raney nickel joins in the continuous fully mixed flow reactor;
[0050] S2. Introduce nitrogen gas into the continuous fully mixed flow reactor, then circulate hydrogen gas to replace and raise the pressure to 2.0MPa, and raise the temperature to 120°C;
[0051] S3. Continuously input dinitrobenzene into the continuous fully mixed flow reactor to carry out hydrogenation reaction;
[0052] S4. The phenylenediamine reducing solution generate...
Embodiment 2
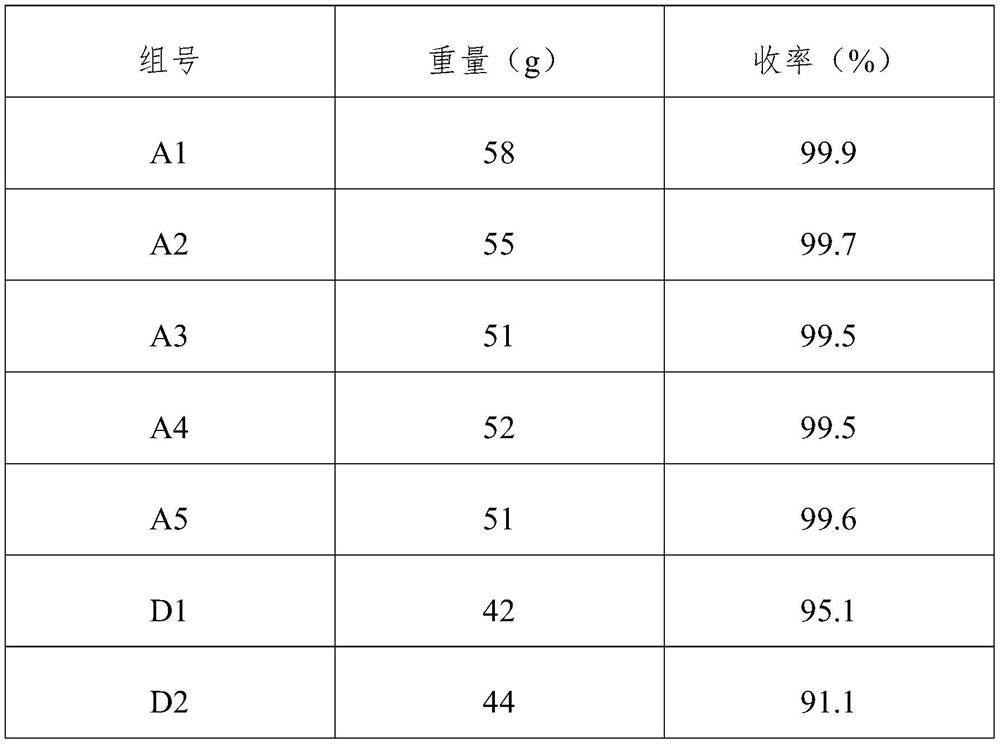
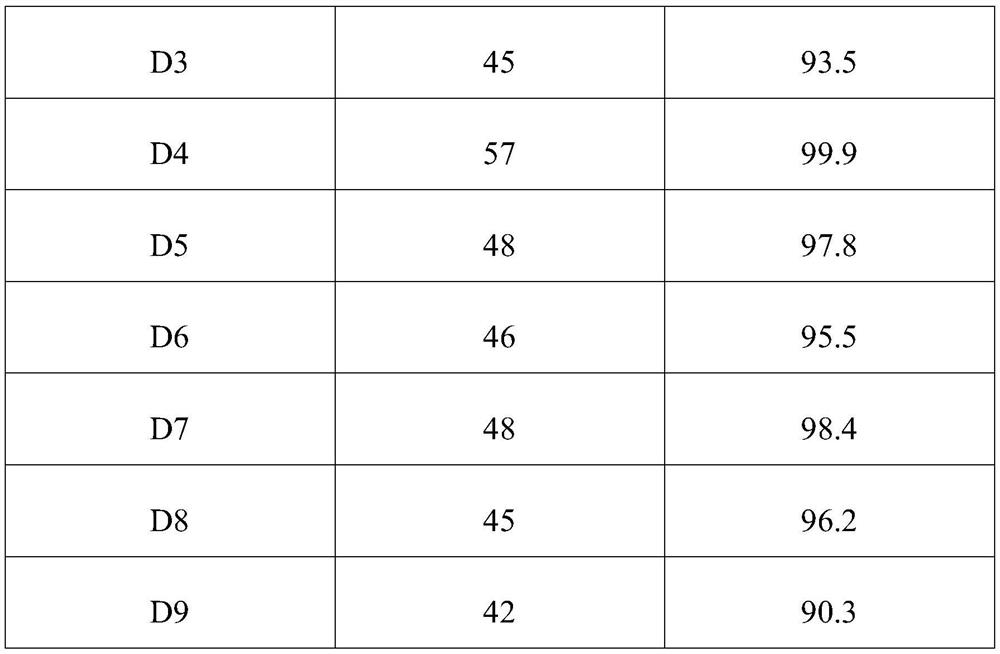
[0058] As another embodiment, all the other features are the same as in Example 1, except that the addition of phenylenediamine is 90 grams.
[0059] In this example, 1 hour was taken as the experiment time, and the phenylenediamine A2 generated during the experiment time was collected, and the weight and yield of phenylenediamine were detected and recorded. See Table 1 for relevant data.
Embodiment 3
[0061] As another embodiment, all the other features are the same as in Example 1, except that the added amount of phenylenediamine is 110 grams.
[0062]In this example, 1 hour was taken as the experiment time, and the phenylenediamine A3 generated during the experiment time was collected, and the weight and yield of phenylenediamine were detected and recorded. See Table 1 for relevant data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com