Lycorine nanoparticle as well as preparation method and application thereof
A nanoparticle and lycorine hydrochloride technology, which is applied in nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of high quality requirements, high production risk, and high price, and achieve high mechanical strength, suitable mechanical properties, and stability good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
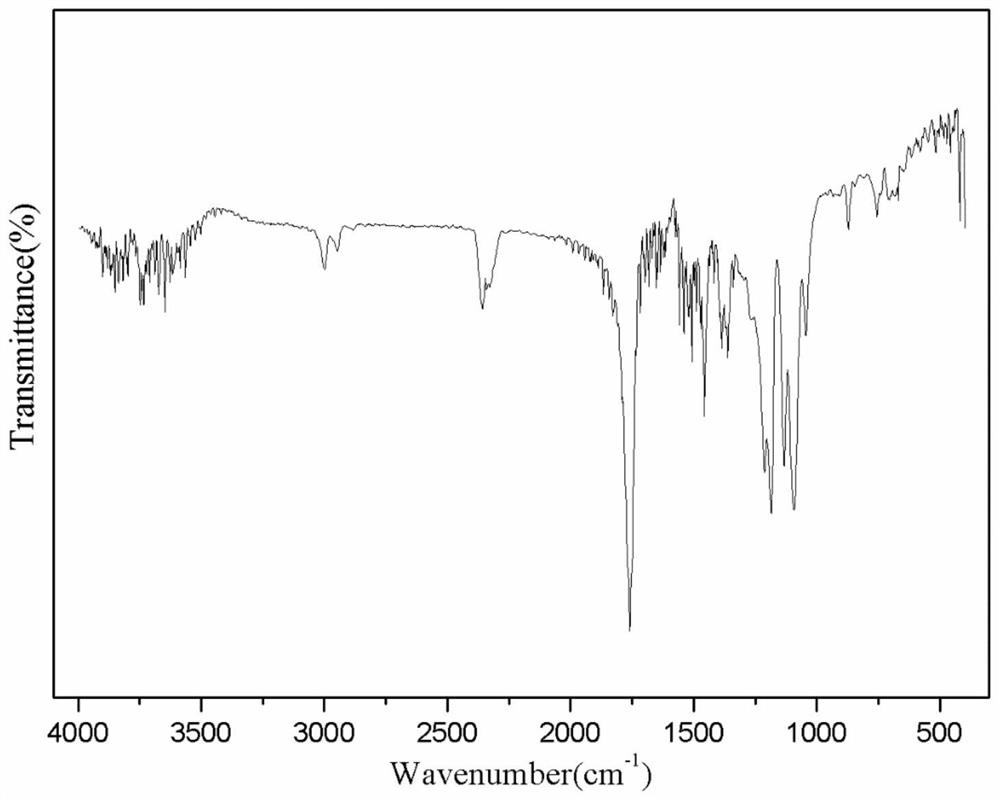
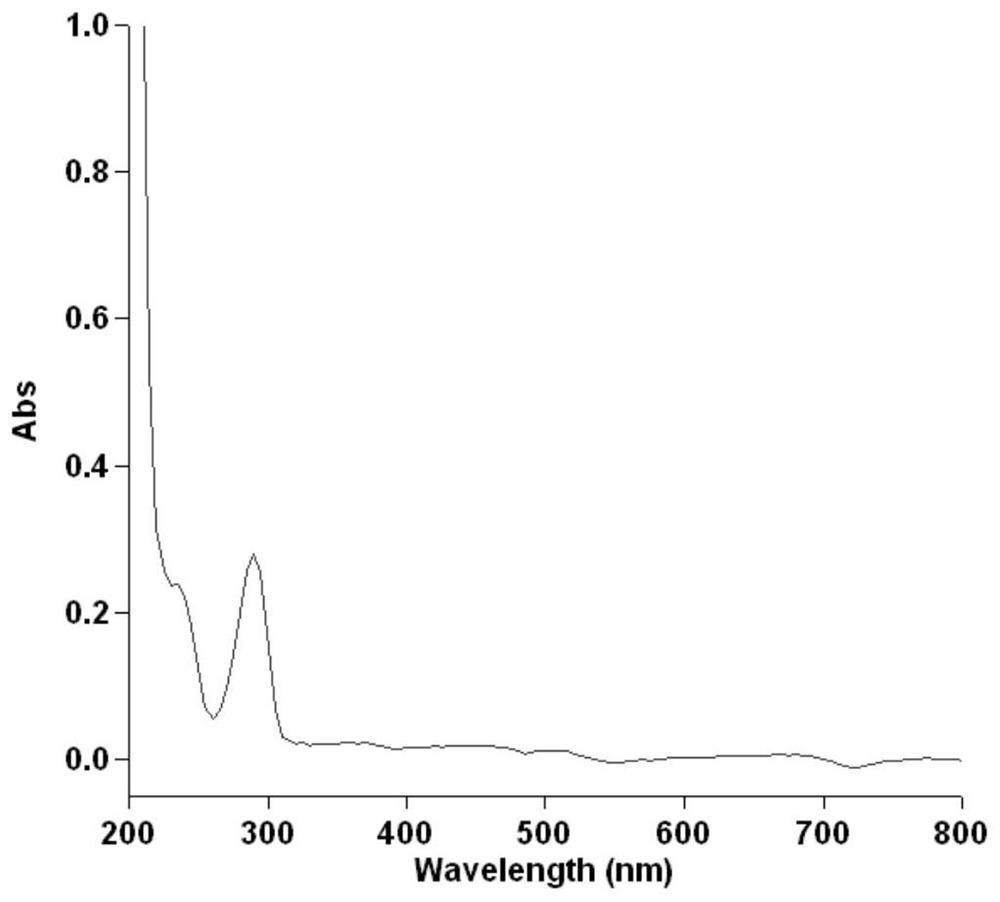
Image
Examples
Embodiment 1
[0057] Preparation of Lycorine Hydrochloride Nanoparticles:
[0058] (1) Purification of DL lactide
[0059] Take about 4 g of lactide powder, add 30 mL of ethyl acetate solution to the beaker, pour the obtained lactide into the beaker, stir to dissolve in a water bath at a temperature of 95 ° C, and then cool it at room temperature, After the temperature dropped to room temperature, it was stored in a refrigerator at 4°C, filtered after 2 hours, and dried in a vacuum drying box for 36 hours to obtain the crystal powder of lactide. The above experiment was repeated twice.
[0060] (2) Preparation of racemic polylactic acid
[0061] Weigh an appropriate amount of recrystallized lactide as a raw material, stannous octoate as a catalyst, and an appropriate amount of water as an initiator. The oil bath was heated to 120°C, and the reaction was carried out for 3 hours under stirring. Dichloromethane was added to the product obtained by the reaction to speed up the dissolution, an...
Embodiment 2
[0068] The preparation method is the same as in Example 1, and the difference is:
[0069] In (i) of step (3), the consumption of racemic polylactic acid is 15mg, and the consumption of lycorine hydrochloride is 6mg; ultrasonic power is 200w;
[0070] The concentration of SDS in (ii) of step (3) becomes 2%; ultrasonic power is 40W;
Embodiment 3
[0072] The preparation method is the same as in Example 1, and the difference is:
[0073] In (i) of step (3), the consumption of racemic polylactic acid is 30mg, the consumption of lycorine hydrochloride is 12mg, and the ultrasonic power is 220w;
[0074] The concentration of SDS in (ii) of step (3) was changed to 3%; the ultrasonic power was 50W.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com