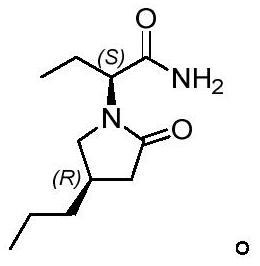
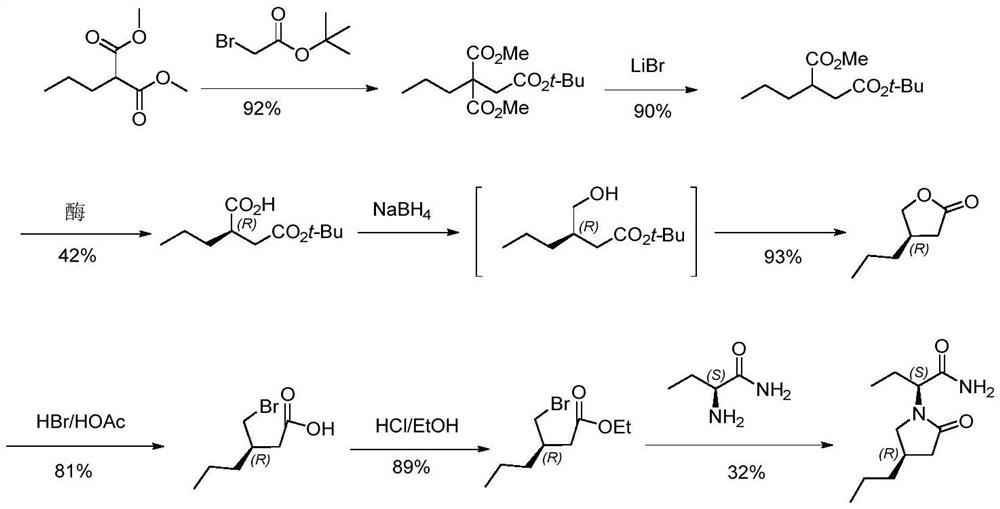
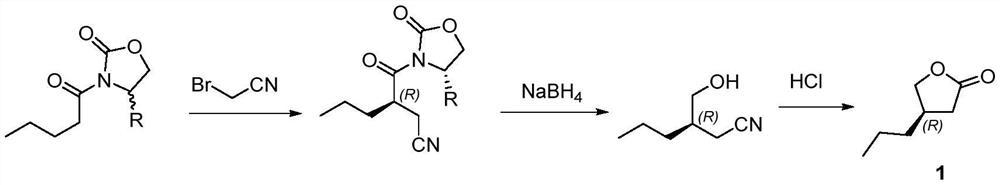
A kind of preparation method of buvaracetam
A compound, methanol technology, applied in organic chemistry methods, drug combinations, organic chemistry and other directions, can solve the problems of low yield of β-propyl butyrolactone, low atom economy, high comprehensive production cost, and achieve high yield High, highlighting the advantages of step economy and the effect of step economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of compound 3
[0061]
[0062] Put compound 2 (130g, 0.61mol, 1.0eq), LiBr (53g, 0.61mol, 1.0eq), DMF (650mL) and water (22.0g, 1.22mol, 2.0eq) into a 1L four-necked flask, and heat up to 130~ 140°C, keep warm for 4 hours, after the reaction is complete, cool down to 10-20°C, add saturated NH at 10-20°C 4 Cl aqueous solution (260mL), toluene (780mL) were stirred for 30 minutes, and the layers were allowed to stand. The aqueous layer was washed once with toluene (400mL). After drying with anhydrous sodium sulfate, the solvent was removed to dryness under reduced pressure to obtain 86.7 g of compound 3 as a colorless oil, with a yield of 92%. ESI-HRMS(m / z):C 8 h 14 NO 2 [M+H + ]Theoretical calculation value: 156.1019, measured value: 154.1023; 1 HNMR (400MHz, d 6 -Acetone) δ3.70(s,3H),2.82(m,1H),2.73(m,2H),1.70-1.62(m,2H),1.36(m,2H),0.92(t,J=7.2Hz ,3H); 13 CNMR (100Hz, d 6 -Acetone) δ174.1, 118.9, 52.3, 41.9, 34.2, 20.4, 14.1.
Embodiment 2
[0063] Embodiment 2: the synthesis of compound 4
[0064]
[0065] Put tris(hydroxymethyl)aminomethane (2.7g) and water (720mL) into a 2L four-necked bottle, start stirring and adjust the pH to about 8.1 with 1.0M HCl, add porcine pancreatic lipase (45.0g), and heat up to 28 ~33°C, add compound 3 (90.0g, 0.58mol, 1.0eq) prepared according to the method in Example 1, THF (90mL), control the temperature at 28~33°C, adjust the pH to 8.0~8.1 with 1.0M NaOH solution, and keep warm After 14 to 18 hours, the reaction is complete, add diatomaceous earth (45.0g) and stir for 30 minutes, filter, filter the residue with 450mL ethyl acetate, and filter, combine the filtrate to separate layers, wash the water layer with ethyl acetate 3 times, each time 450mL acetic acid Ethyl ester, the water layer was cooled to 0-5°C, and 1.0M H 2 SO 4 (248g) to adjust the pH to 1.9-2.1, after the adjustment, the temperature was raised to 20-25°C, 45g of diatomaceous earth was added, 900mL of ethyl a...
Embodiment 3
[0066] Embodiment 3: the synthesis of compound 5
[0067]
[0068] Put compound 4 (32.0g, 0.23mol, 1.0eq), methanol (128mL), refined 30% hydrochloric acid aqueous solution (5.8g, 0.058mol, 0.25eq) into a 250ml four-necked bottle, heat up to 35-45°C for 20 hours After the reaction is complete, remove the methanol under reduced pressure at 40-45°C, add 320mL of dichloromethane and 320ml of water after dehydration, stir for 10 minutes, let stand to separate and wash the water layer once with 1600mL of dichloromethane, let stand and separate , combined the organic layers, the organic layer was washed twice with saturated NaCl aqueous solution, and the organic layer was washed with 320ml saturated NaHCO 3 After the aqueous solution was washed once, the mixture was left to stand and separated, and the organic layer was dried over anhydrous sodium sulfate and desolvated under reduced pressure to obtain 32.6 g of compound 5 as a colorless oil, with a yield of 93%. ESI-HRMS(m / z):C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com