Microbial limit inspection method for coptis chinensis formula granules
A technology of microbial limit and formula granules, applied in the field of microbial limit inspection of Coptis chinensis formula granules, can solve the problems of inability to accurately detect the microbial limit of Coptis chinensis, unpublished and other problems, and achieves the reduction of complexity, the number of washings, and the use of reagents. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
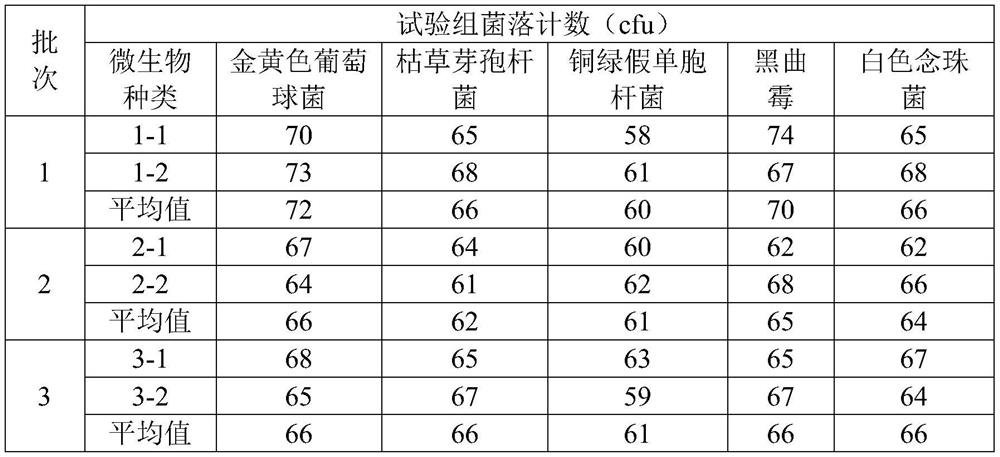
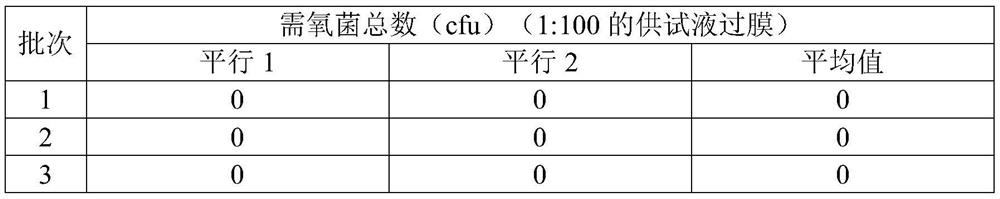
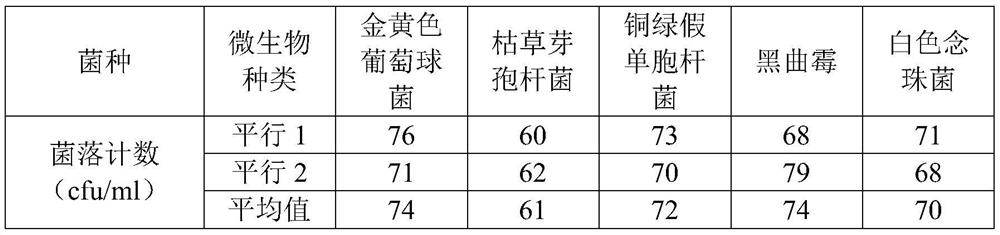
[0053] The invention adopts the diluent added with histidine to dissolve the sample, and uses the membrane filtration method in combination to detect the microbes of the Coptis rhizome formula granules.
[0054] (1) Preparation of test solution
[0055] Preparation of bacterial solution
[0056] (1) Preparation of bacterial suspensions of Staphylococcus aureus, Pseudomonas aeruginosa, and Bacillus subtilis, inoculate a bacterium in 10ml of tryptone soy-ptone liquid medium, cultivate it at 33°C for 21 hours, and then use pH7. 0 sterile sodium chloride-peptone buffer to make a bacterial suspension with a bacterial count of 10-100cfu per 1ml. The selected bacteria were Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis.
[0057] (2) Preparation of Candida albicans suspension
[0058] Inoculate the fresh culture of Candida albicans in 10ml Sabouraud dextrose liquid medium, cultivate it at 23°C for 22 hours, and use pH 7.0 sterile sodium chloride-peptone buffer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com